Information
Authors: Jenny Volstorf, Maria Filipa Castanheira
Version: C | 2.1Published: 2024-12-31
- minor editorial changes plus new side note "Commercial relevance"
- profile update resulting in major editorial and content changes (changing the scoring in criteria 1-5, 7, and 10)
- transfer to consistent age class and label structure resulting in changed appearance
WelfareScore | farm
The score card gives our welfare assessments for aquatic species in 10 criteria.
For each criterion, we score the probability to experience good welfare under minimal farming conditions ("Likelihood") and under high-standard farming conditions ("Potential") representing the worst and best case scenario. The third dimension scores how certain we are of our assessments based on the number and quality of sources we found ("Certainty").
The WelfareScore sums just the "High" scores in each dimension. Although good welfare ("High") seems not possible in some criteria, there could be at least a potential improvement from low to medium welfare (indicated by ➚ and the number of criteria).
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
➚ = potential improvements not reaching "High" - Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10 per dimension)
General remarks
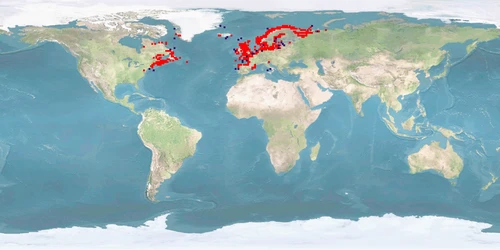
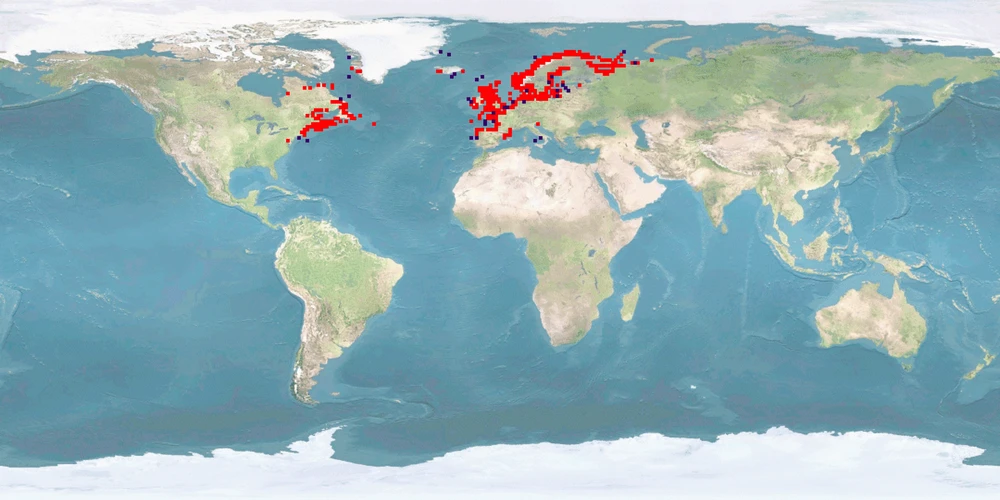
Salmo salar is a salmonid from both coasts of the northern Atlantic, migrating into boardering rivers to spawn. It is the most frequently farmed fish in Europe which represents 50% of the worldwide S. salar production. Upbringing in fresh water, predominantly in flow-through tanks, lasts for 1-1.5 years after which smoltification prepares the IND for life in seawater; on-growing in sea cages covers 50-70% of the life cycle. Where the culture site does not provide suitable conditions for sea transfer, IND are grown out in land-based freshwater RAS to harvestable size. IND are slaughtered before reaching maturity. ADULTS destined to become broodstock are transferred back to fresh water about 2 months before spawning.
The underlying migration habit is one of the factors very hard to accommodate in captivity, as it is unclear whether it is sufficient to provide the species with the conditions of the respective life stages or whether it needs to experience the transition. Other factors responsible for the low WelfareScore are substrate needs as well as high levels of aggression, stress, and deformations under farming conditions. Avoiding manipulation to induce spawning, providing substrate, and applying the high-standard slaughter methods are ways towards improving welfare for S. salar in captivity.
Note: LARVAE are called ALEVINS; JUVENILES are called PARR in fresh water and SMOLTS after bodily modification to sustain seawater; SPAWNERS are called GRILSE at first spawning and KELTS thereafter (very rare). We added an age class “Adults” for the case when it is not clear whether SMOLTS or GRILSE are meant. As IND are usually slaughtered before reaching maturity, the age class “ADULTS” in farms refers to ADULTS to become SPAWNERS, i.e., during holding before/between the spawning event(s).
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
There are unclear findings for minimal and high-standard farming conditions, as the missing wild information at sea does not allow a comparison with farming conditions. Our conclusion is based on a medium amount of evidence.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal farming conditions, as some trays and tanks do not cover the whole range in the wild. It is medium for high-standard farming conditions, as other trays and tanks cover the range in the wild, although we cannot be sure in GRILSE and KELTS. Our conclusion is based on a medium amount of evidence, as farm information for GRILSE and KELTS is missing.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal farming conditions, as both strains undertake more or less extensive migrations, and we cannot be sure that providing each age class with their respective environmental conditions will satisfy their urge to migrate or whether they need to experience the transition. It is medium for high-standard farming conditions, as the non-ANADROMOUS strain at least does not migrate between sea- and fresh water and the range in captivity potentially overlaps with the migration distance (although unknown). Our conclusion is based on a medium amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of these circumstances?
It is low for minimal farming conditions, as the species is manipulated (wild breeders, separation by sex, hormonal/photoperiod/temperature manipulation, stripping). It is medium for high-standard farming conditions, as omitting hormonal manipulation and stripping is easily imaginable but needs verification for the farming context. Our conclusion is based on a medium amount of evidence.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is low for minimal farming conditions, as – even in the absence of density data in the wild – we may conclude from studies in farms that densities in some tanks are potentially stress inducing. It is medium for high-standard farming conditions, as densities around 20 kg/m3 in cages increase welfare. Our conclusion is based on a medium amount of evidence, as wild information for SMOLTS, GRILSE, and KELTS is missing


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal farming conditions, as – even in the absence of aggression data in the wild – we may conclude from studies in labs that some densities and current velocities potentially induce aggression. It is medium for high-standard farming conditions, as innovations to decrease aggression by decreasing density or increasing current velocity need to be verified for the farming context. Our conclusion is based on a medium amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions, as the species uses substrate, but many or all farming facilities for each age class are devoid of it. It is medium for high-standard farming conditions a) given hatching substrate for ALEVINS as well as cover and enrichment for PARR in tanks, b) as improvements for GRILSE and KELTS are unlikely, and c) as innovations for enrichment need to be verified for the farming context. Our conclusion is based on a medium amount of evidence, as innovations for cages are missing, for example.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions. It is medium for high-standard farming conditions, as some innovations to reduce stress need to be verified for the farming context. Our conclusion is based on a medium amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is low for minimal farming conditions, as malformation rates exceed 10%. It is medium for high-standard farming conditions, as some malformations result from conditions that may be changed (handling, heritability). Our conclusion is based on a medium amount of evidence, as improvement of the situation by adjusting conditions needs more proof.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is low for minimal farming conditions. It is high for high-standard farming conditions, as percussive stunning or electrical stunning, each followed by exsanguination, induce unconsciousness fast, kill while still unconscious, and are verified for the farming context. Our conclusions are based on a medium amount of evidence, as a reproducible set of rules for electrical stunning is missing.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 5 90, fully domesticated. Cultured since 19th century 9.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes:
- WILD: carnivorous 91 92 93.
- FARM: no data found yet.
- LAB: SMOLTS: fish oil may be partly* replaced by sustainable sources 94. Fish meal may be partly* replaced by sustainable sources parallel to fish oil being mostly* replaced 95.
* partly = <51% – mostly = 51-99% – completely = 100%
Side note: Commercial relevance
How much is this species farmed annually?
2,615,962 t/year 1990-2019 amounting to estimated 411,000,000-554,000,000 IND/year 1990-2019 96.
Glossary
ALEVINS = larvae until the end of yolk sac absorption
ANADROMOUS = migrating from the sea into fresh water to spawn
DOMESTICATION LEVEL 5 = selective breeding programmes are used focusing on specific goals 90
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FRY = larvae from external feeding on
GRILSE = adults returning from sea to home river to spawn
IND = individuals
JUVENILES = fully developed but immature individuals
KELTS = adults surviving spawning
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening
PARR = juvenile stage in rivers
PELAGIC = living independent of bottom and shore of a body of water
PHOTOPERIOD = duration of daylight
RAS = Recirculating Aquaculture System - almost completely closed system using filters to clean and recirculate water with the aim of reducing water input and with the advantage of enabling close control of environmental parameters to maintain high water quality
SMOLTS = juvenile stage migrating to the sea
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
WILD = setting in the wild
Bibliography
2 Gustafson-Greenwood, Karla I., and John R. Moring. 1990. Territory size and distribution of newly-emerged Atlantic salmon (Salmo salar). Hydrobiologia 206: 125–131. https://doi.org/10.1007/BF00018638.
3 EFSA. 2008. Animal welfare aspects of husbandry systems for farmed Atlantic salmon - Scientific Opinion of the Panel on Animal Health and Welfare. European Food Safety Authority. EFSA Journal 6: 1–31. https://doi.org/10.2903/j.efsa.2008.736.
4 Hesthagen, T. 1990. Home range of juvenile Atlantic salmon, Salmo salar, and brown trout, Salmo trutta, in a Norwegian stream. Freshwater Biology 24: 63–67. https://doi.org/10.1111/j.1365-2427.1990.tb00307.x.
5 Girard, Isabelle L, James W.A Grant, and Stefán Ó Steingrímsson. 2004. Foraging, growth, and loss rate of young-of-the-year Atlantic salmon (Salmo salar) in relation to habitat use in Catamaran Brook, New Brunswick. Canadian Journal of Fisheries and Aquatic Sciences 61: 2339–2349. https://doi.org/10.1139/f04-216.
6 Roussel, Jean-Marc, Richard A. Cunjak, Robert Newbury, Daniel Caissie, and Alexander Haro. 2004. Movements and habitat use by PIT-tagged Atlantic salmon parr in early winter: the influence of anchor ice. Freshwater Biology 49: 1026–1035. https://doi.org/10.1111/j.1365-2427.2004.01246.x.
7 Davidsen, Jan G., Linda Eikås, Richard D. Hedger, Eva B. Thorstad, Lars Rønning, Aslak D. Sjursen, Ole K. Berg, Gunnbjørn Bremset, Sten Karlsson, and Line E. Sundt-Hansen. 2020. Migration and habitat use of the landlocked riverine Atlantic salmon Salmo salar småblank. Hydrobiologia 847: 2295–2306. https://doi.org/10.1007/s10750-020-04254-6.
8 Summerfelt, Steven T., Frode Mathisen, Astrid Buran Holan, and Bendik Fyhn Terjesen. 2016. Survey of large circular and octagonal tanks operated at Norwegian commercial smolt and post-smolt sites. Aquacultural Engineering 74: 105–110. https://doi.org/10.1016/j.aquaeng.2016.07.004.
9 Jones, M. 2004. Cultured Aquatic Species Information Programme. Salmo salar. Rome: FAO Fisheries and Aquaculture Department.
10 Cubitt, K Fiona, Svante Winberg, Felicity A Huntingford, Sunil Kadri, Vivian O Crampton, and Øyvind Øverli. 2008. Social hierarchies, growth and brain serotonin metabolism in Atlantic salmon (Salmo salar) kept under commercial rearing conditions. Physiology & behavior 94: 529–35. https://doi.org/10.1016/j.physbeh.2008.03.009.
11 Dempster, Tim, Jon-Erik Juell, Jan Erik Fosseidengen, Arne Fredheim, and Pål Lader. 2008. Behaviour and growth of Atlantic salmon (Salmo salar L.) subjected to short-term submergence in commercial scale sea-cages. Aquaculture 276: 103–111. https://doi.org/10.1016/j.aquaculture.2008.01.018.
12 Noble, Chris, Kristine Gismervik, Martin Haugmo Iversen, Jelena Kolarevic, Jonatan Nilsson, Lars Helge Stien, and James F Turnbull. 2018. Welfare indicators for farmed Atlantic salmon: Tools for assessing fish welfare.
13 Crisp, D. T., and P. A. Carling. 1989. Observations on siting, dimensions and structure of salmonid redds. Journal of Fish Biology 34: 119–134. https://doi.org/10.1111/j.1095-8649.1989.tb02962.x.
14 Baglinière, J. L., G. Maisse, and A. Nihouarn. 1990. Migratory and reproductive behaviour of female adult Atlantic salmon, Salmo salar L., in a spawning stream. Journal of Fish Biology 36: 511–520. https://doi.org/10.1111/j.1095-8649.1990.tb03553.x.
15 Rimmer, D. M., U. Paim, and R. L. Saunders. 1984. Changes in the Selection of Microhabitat by Juvenile Atlantic Salmon (Salmo salar) at the Summer–Autumn Transition in a Small River. Canadian Journal of Fisheries and Aquatic Sciences 41: 469–475. https://doi.org/10.1139/f84-056.
16 Degraaf, D. A., and L. H. Bain. 1986. Habitat Use by and Preferences of Juvenile Atlantic Salmon in Two Newfoundland Rivers. Transactions of the American Fisheries Society 115: 671–681. https://doi.org/10.1577/1548-8659(1986)115<671:HUBAPO>2.0.CO;2.
17 Morantz, D. L., R. K. Sweeney, C. S. Shirvell, and D. A. Longard. 1987. Selection of Microhabitat in Summer by Juvenile Atlantic Salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 44: 120–129. https://doi.org/10.1139/f87-015.
18 Kennedy, G. J. A., and C. D. Strange. 1982. The distribution of salmonids in upland streams in relation to depth and gradient. Journal of Fish Biology 20: 579–591. https://doi.org/10.1111/j.1095-8649.1982.tb03956.x.
19 Stead, Selina M., and Lindsay Laird. 2002. The Handbook of Salmon Farming. Food Sciences. London: Springer-Verlag.
20 Holm, M., J. Chr Holst, and L. P. Hansen. 2000. Spatial and temporal distribution of post-smolts of Atlantic salmon (Salmo salar L.) in the Norwegian Sea and adjacent areas. ICES Journal of Marine Science: Journal du Conseil 57: 955–964. https://doi.org/10.1006/jmsc.2000.0700.
21 Davidsen, J. G., N. Plantalech Manel-la, F. Økland, O. H. Diserud, E. B. Thorstad, B. Finstad, R. Sivertsgård, R. S. McKinley, and A. H. Rikardsen. 2008. Changes in swimming depths of Atlantic salmon Salmo salar post-smolts relative to light intensity. Journal of Fish Biology 73: 1065–1074. https://doi.org/10.1111/j.1095-8649.2008.02004.x.
22 Johansson, David, Kari Ruohonen, Jon-Erik Juell, and Frode Oppedal. 2009. Swimming depth and thermal history of individual Atlantic salmon (Salmo salar L.) in production cages under different ambient temperature conditions. Aquaculture 290: 296–303. https://doi.org/10.1016/j.aquaculture.2009.02.022.
23 Beland, Kenneth F., Richard M. Jordan, and Alfred L. Meister. 1982. Water Depth and Velocity Preferences of Spawning Atlantic Salmon in Maine Rivers. North American Journal of Fisheries Management 2: 11–13. https://doi.org/10.1577/1548-8659(1982)2<11:WDAVPO>2.0.CO;2.
24 Moir, H. J., C. Soulsby, and A. Youngson. 1998. Hydraulic and sedimentary characteristics of habitat utilized by Atlantic salmon for spawning in the Girnock Burn, Scotland. Fisheries Management and Ecology 5: 241–254. https://doi.org/10.1046/j.1365-2400.1998.00105.x.
25 Soulsby, C., A. F. Youngson, H. J. Moir, and I. A. Malcolm. 2001. Fine sediment influence on salmonid spawning habitat in a lowland agricultural stream: a preliminary assessment. Science of The Total Environment 265: 295–307. https://doi.org/10.1016/S0048-9697(00)00672-0.
26 Hubley, P. Bradford, Peter G. Amiro, A. Jamie F. Gibson, Gilles L. Lacroix, and Anna M. Redden. 2008. Survival and behaviour of migrating Atlantic salmon (Salmo salar L.) kelts in river, estuarine, and coastal habitat. ICES Journal of Marine Science: Journal du Conseil 65: 1626–1634. https://doi.org/10.1093/icesjms/fsn129.
27 Halttunen, Elina, Audun H. Rikardsen, Jan G. Davidsen, Eva B. Thorstad, and J. Brian Dempson. 2009. Survival, Migration Speed and Swimming Depth of Atlantic Salmon Kelts During Sea Entry and Fjord Migration. In Tagging and Tracking of Marine Animals with Electronic Devices, ed. Jennifer L. Nielsen, Haritz Arrizabalaga, Nuno Fragoso, Alistair Hobday, Molly Lutcavage, and John Sibert, 35–49. Reviews: Methods and Technologies in Fish Biology and Fisheries 9. Springer Netherlands.
28 Thorpe, J. E. 1994. Reproductive strategies in Atlantic salmon, Salmo salar L. Aquaculture Research 25: 77–87. https://doi.org/10.1111/j.1365-2109.1994.tb00668.x.
29 Moore, A., E. C. E. Potter, N. J. Milner, and S. Bamber. 1995. The migratory behaviour of wild Atlantic salmon (Salmo salar) smolts in the estuary of the River Conwy, North Wales. Canadian Journal of Fisheries and Aquatic Sciences 52: 1923–1935. https://doi.org/10.1139/f95-784.
30 Armstrong, John D, James WA Grant, Harvey L Forsgren, Kurt D Fausch, Richard M DeGraaf, Ian A Fleming, Terry D Prowse, and Isaac J Schlosser. 1998. The application of science to the management of Atlantic salmon (Salmo salar): integration across scales. Canadian Journal of Fisheries and Aquatic Sciences 55: 303–311. https://doi.org/10.1139/d98-014.
31 Cunjak, R. A., and J. Therrien. 1998. Inter-stage survival of wild juvenile Atlantic salmon, Salmo salar L. Fisheries Management and Ecology 5: 209–223. https://doi.org/10.1046/j.1365-2400.1998.00094.x.
32 Hedger, Richard D., Francois Martin, Daniel Hatin, Francois Caron, F. G. Whoriskey, and Julian J. Dodson. 2008. Active migration of wild Atlantic salmon Salmo salar smolt through a coastal embayment. ResearchGate 355: 235–246. https://doi.org/10.3354/meps07239.
33 Sutterlin, A. M., and D. MacLean. 1984. Age at First Maturity and the Early Expression of Oocyte Recruitment Processes in Two Forms of Atlantic Salmon (Salmo salar) and Their Hybrids. Canadian Journal of Fisheries and Aquatic Sciences 41: 1139–1149. https://doi.org/10.1139/f84-135.
34 Hutchings, Jeffrey A. 1986. Lakeward Migrations by Juvenile Atlantic Salmon, Salmo salar. Canadian Journal of Fisheries and Aquatic Sciences 43: 732–741. https://doi.org/10.1139/f86-090.
35 McCormick, Stephen D, Lars P Hansen, Thomas P Quinn, and Richard L Saunders. 1998. Movement, migration, and smolting of Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 55: 77–92. https://doi.org/10.1139/d98-011.
36 Erkinaro, J. 1995. The age structure and distribution of Atlantic salmon parr, Salmo salar L., in small tributaries and main stems of the subarctic River Teno, northern Finland. Ecology of Freshwater Fish 4: 53–61. https://doi.org/10.1111/j.1600-0633.1995.tb00117.x.
37 Carr, J. W., J. M. Anderson, F. G. Whoriskey, and T. Dilworth. 1997. The occurrence and spawning of cultured Atlantic salmon (Salmo salar) in a Canadian river. ICES Journal of Marine Science: Journal du Conseil 54: 1064–1073. https://doi.org/10.1016/S1054-3139(97)80010-0.
38 Newman, Rhian C., Tim Ellis, Phil I. Davison, Mark J. Ives, Rob J. Thomas, Sian W. Griffiths, and William D. Riley. 2015. Using novel methodologies to examine the impact of artificial light at night on the cortisol stress response in dispersing Atlantic salmon (Salmo salar L.) fry. Conservation Physiology 3: cov051. https://doi.org/10.1093/conphys/cov051.
39 Hutchings, Jeffrey A, and Megan EB Jones. 1998. Life history variation and growth rate thresholds for maturity in Atlantic salmon, Salmo salar. Canadian Journal of Fisheries and Aquatic Sciences 55: 22–47. https://doi.org/10.1139/d98-004.
40 Guay, J C, D Boisclair, M Leclerc, and M Lapointe. 2003. Assessment of the transferability of biological habitat models for Atlantic salmon parr (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 60: 1398–1408. https://doi.org/10.1139/f03-120.
41 Bui, Samantha, Frode Oppedal, Øyvind J. Korsøen, Damien Sonny, and Tim Dempster. 2013. Group Behavioural Responses of Atlantic Salmon (Salmo salar L.) to Light, Infrasound and Sound Stimuli. Edited by Josep V. Planas. PLoS ONE 8: e63696. https://doi.org/10.1371/journal.pone.0063696.
42 Myers, Ransom A., Jeffrey A. Hutchings, and R. John Gibson. 1986. Variation in Male Parr Maturation Within and Among Populations of Atlantic Salmon, Salmo salar. Canadian Journal of Fisheries and Aquatic Sciences 43: 1242–1248. https://doi.org/10.1139/f86-154.
43 Metcalfe, Neil B. 1998. The interaction between behavior and physiology in determining life history patterns in Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 55: 93–103. https://doi.org/10.1139/d98-005.
44 Sambraus, F., K. A. Glover, T. Hansen, T. W. K. Fraser, M. F. Solberg, and P. G. Fjelldal. 2014. Vertebra deformities in wild Atlantic salmon caught in the Figgjo River, southwest Norway. Journal of Applied Ichthyology 30: 777–782. https://doi.org/10.1111/jai.12517.
45 Lindberg, Dan-Erik. 2011. Atlantic salmon (Salmo salar) migration behavior and preferences in smolts, spawners and kelts. Report 14. Umeå Swedish University of Agricultural Sciences: Department of Wildlife, Fish and Environmental Studies.
46 Hutchings, Jeffrey A., William R. Ardren, Bjørn T. Barlaup, Eva Bergman, Keith D. Clarke, Larry A. Greenberg, Colin Lake, et al. 2019. Life-history variability and conservation status of landlocked Atlantic salmon: an overview. Canadian Journal of Fisheries and Aquatic Sciences 76: 1697–1708. https://doi.org/10.1139/cjfas-2018-0413.
47 Jones, J. W. 1959. The salmon. London: Collins.
48 Fleming, Ian A., and Sigurd Einum. 2010. Reproductive Ecology: A Tale of Two Sexes. In Atlantic Salmon Ecology, 33–65. John Wiley & Sons, Ltd. https://doi.org/10.1002/9781444327755.ch2.
49 De Gaudemar, Benoit, and Edward Beall. 1999. Reproductive behavioural sequences of single pairs of Atlantic salmon in an experimental stream. Animal Behaviour 57: 1207–1217. https://doi.org/10.1006/anbe.1999.1104.
50 De Gaudemar, B., J. M. Bonzom, and E. Beall. 2000. Effects of courtship and relative mate size on sexual motivation in Atlantic salmon. Journal of Fish Biology 57: 502–515. https://doi.org/10.1111/j.1095-8649.2000.tb02188.x.
51 Järvi, Torbjörn. 1990. The Effects of Male Dominance, Secondary Sexual Characteristics and Female Mate Choice on the Mating Success of Male Atlantic Salmon Salmo salar. Ethology 84: 123–132. https://doi.org/10.1111/j.1439-0310.1990.tb00789.x.
52 Dill, Peter A. 1977. Development of behaviour in alevins of atlantic salmon, Salmo salar, and rainbow trout, S. gairdneri. Animal Behaviour 25, Part 1: 116–121. https://doi.org/10.1016/0003-3472(77)90073-2.
53 Gibson, R. J. 1983. Water velocity as a factor in the change from aggressive to schooling behaviour and subsequent migration of Atlantic salmon smolt (Salmo salar). Le Naturaliste canadien.
54 Riley, W. D., A. T. Ibbotson, D. L. Maxwell, P. I. Davison, W. R. C. Beaumont, and M. J. Ives. 2014. Development of schooling behaviour during the downstream migration of Atlantic salmon Salmo salar smolts in a chalk stream. Journal of Fish Biology 85: 1042–1059. https://doi.org/10.1111/jfb.12457.
55 Hosfeld, Camilla Diesen, Jannicke Hammer, Sigurd O. Handeland, Sveinung Fivelstad, and Sigurd O. Stefansson. 2009. Effects of fish density on growth and smoltification in intensive production of Atlantic salmon (Salmo salar L.). Aquaculture 294: 236–241. https://doi.org/10.1016/j.aquaculture.2009.06.003.
56 Turnbull, James, Alisdair Bell, Colin Adams, James Bron, and Felicity Huntingford. 2005. Stocking density and welfare of cage farmed Atlantic salmon: application of a multivariate analysis. Aquaculture 243: 121–132. https://doi.org/10.1016/j.aquaculture.2004.09.022.
57 Johansson, David, Kari Ruohonen, Anders Kiessling, Frode Oppedal, Jan-Erik Stiansen, Mark Kelly, and Jon-Erik Juell. 2006. Effect of environmental factors on swimming depth preferences of Atlantic salmon (Salmo salar L.) and temporal and spatial variations in oxygen levels in sea cages at a fjord site. Aquaculture 254: 594–605. https://doi.org/10.1016/j.aquaculture.2005.10.029.
58 Adams, C E, J F Turnbull, A Bell, J E Bron, and F A Huntingford. 2007. Multiple determinants of welfare in farmed fish: stocking density, disturbance, and aggression in Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 64: 336–344. https://doi.org/10.1139/f07-018.
59 Cañon Jones, Hernán Alberto, Chris Noble, Børge Damsgård, and Gareth P. Pearce. 2011. Social network analysis of the behavioural interactions that influence the development of fin damage in Atlantic salmon parr (Salmo salar) held at different stocking densities. Applied Animal Behaviour Science 133: 117–126. https://doi.org/10.1016/j.applanim.2011.05.005.
60 Anon. 2004. Forskrift om drift av akvakulturanlegg (akvakulturdriftsforskriften). Fastsatt av Fiskeri- og kystdepartementet 22. desember 2004 med hjemmel i lov 14. juni 1985 nr. 68 om oppdrett av fisk, skalldyr, lov 19. desember 2003 nr. 124 om matproduksjon og mattrygghet og lov 20. desember 1974 nr. 73 om dyrevern.
61 Metcalfe, N. B., and J. E. Thorpe. 1992. Early predictors of life-history events: the link between first feeding date, dominance and seaward migration in Atlantic salmon, Salmo salar L. Journal of Fish Biology 41: 93–99. https://doi.org/10.1111/j.1095-8649.1992.tb03871.x.
62 Vaz-Serrano, J., M. L. Ruiz-Gomez, H. M. Gjoen, P. V. Skov, F. A. Huntingford, Øyvind Øverli, and E. Höglund. 2011. Consistent boldness behaviour in early emerging fry of domesticated Atlantic salmon (Salmo salar): Decoupling of behavioural and physiological traits of the proactive stress coping style. Physiology & Behavior 103: 359–364. https://doi.org/10.1016/j.physbeh.2011.02.025.
63 Blanchet, S., J. J. Dodson, and S. Brosse. 2006. Influence of habitat structure and fish density on Atlantic salmon Salmo salar L. territorial behaviour. Journal of Fish Biology 68: 951–957. https://doi.org/10.1111/j.0022-1112.2006.00970.x.
64 Hoar, W. S. 1988. 4 The Physiology of Smolting Salmonids. In Fish Physiology, ed. W. S. Hoar and D. J. Randall, 11:275–343. The Physiology of Developing Fish. Academic Press. https://doi.org/10.1016/S1546-5098(08)60216-2.
65 Iwata, Munehico. 1995. Downstream migratory behavior of salmonids and its relationship with cortisol and thyroid hormones: A review. Aquaculture 135. Application of Endocrinology to Pacific Rim Aquaculture: 131–139. https://doi.org/10.1016/0044-8486(95)01000-9.
66 Cutts, C. J., N. B. Betcalfe, and A. C. Caylor. 1998. Aggression and growth depression in juvenile Atlantic salmon: the consequences of individual variation in standard metabolic rate. Journal of Fish Biology 52: 1026–1037. https://doi.org/10.1111/j.1095-8649.1998.tb00601.x.
67 Cañon Jones, Alberto Hernán. 2011. Social network analysis of behavioural interactions influencing the development of fin damage in Atlantic salmon (Salmo salar). Thesis, University of Cambridge.
68 Cañon Jones, Hernán Alberto Cañon, Chris Noble, Børge Damsgård, and Gareth P. Pearce. 2017. Evaluating the effects of a short-term feed restriction period on the behavior and welfare of Atlantic salmon, Salmo salar, parr using social network analysis and fin damage. Journal of the World Aquaculture Society 48: 35–45. https://doi.org/10.1111/jwas.12322.
69 Solstorm, Frida, David Solstorm, Frode Oppedal, Rolf Erik Olsen, Lars Helge Stien, and Anders Fernö. 2016. Not too slow, not too fast: water currents affect group structure, aggression and welfare in post-smolt Atlantic salmon Salmo salar. 1869-215X. https://doi.org/10.3354/aei00178.
70 Julien, H. P., and N. E. Bergeron. 2006. Effect of Fine Sediment Infiltration During the Incubation Period on Atlantic Salmon (Salmo salar) Embryo Survival. Hydrobiologia 563: 61–71. https://doi.org/10.1007/s10750-005-1035-2.
71 Hansen, Tom, and Krisna R. Torrissen. 1985. Artificial hatching substrate and different times of transfer to first feeding: Effect on growth and protease activities of the Atlantic salmon (Salmo salar). Aquaculture 48: 177–188. https://doi.org/10.1016/0044-8486(85)90103-6.
72 Alnes, Ingeborg Bjerkvik, Knut Helge Jensen, Arne Skorping, and Anne Gro Vea Salvanes. 2021. Ontogenetic Change in Behavioral Responses to Structural Enrichment From Fry to Parr in Juvenile Atlantic Salmon (Salmo salar L.). Frontiers in Veterinary Science 8: 638888. https://doi.org/10.3389/fvets.2021.638888.
73 Pickering, A. D., R. Griffiths, and T. G. Pottinger. 1987. A comparison of the effects of overhead cover on the growth, survival and haematology of juvenile Atlantic salmon, Salmo salar L., brown trout, Salmo trutta L., and rainbow trout, Salmo gairdneri Richardson. Aquaculture 66: 109–124. https://doi.org/10.1016/0044-8486(87)90226-2.
74 Karvonen, Anssi, Mariella Aalto-Araneda, Anna-Maija Virtala, Raine Kortet, Perttu Koski, and Pekka Hyva. 2016. Enriched rearing environment and wild genetic background can enhance survival and disease resistance of salmonid fishes during parasite epidemics. Journal of Applied Ecology 53: 213–221.
75 Räihä, Ville, Lotta‐Riina Sundberg, Roghaieh Ashrafi, Pekka Hyvärinen, and Anssi Karvonen. 2019. Rearing background and exposure environment together explain higher survival of aquaculture fish during a bacterial outbreak. Edited by Nessa O’Connor. Journal of Applied Ecology 56: 1741–1750. https://doi.org/10.1111/1365-2664.13393.
76 Salvanes, Anne Gro Vea, Olav Moberg, Lars O. E. Ebbesson, Tom Ole Nilsen, Knut Helge Jensen, and Victoria A. Braithwaite. 2013. Environmental enrichment promotes neural plasticity and cognitive ability in fish. Proceedings of the Royal Society B: Biological Sciences 280: 20131331. https://doi.org/10.1098/rspb.2013.1331.
77 Jones, Misty D., Eric Krebs, Nathan Huysman, Jill M. Voorhees, and Michael E. Barnes. 2019. Rearing Performance of Atlantic Salmon Grown in Circular Tanks with Vertically-Suspended Environmental Enrichment. Open Journal of Animal Sciences 09: 249–257. https://doi.org/10.4236/ojas.2019.92021.
78 Iversen, Martin, Bengt Finstad, Robert S. McKinley, Robert A. Eliassen, Kristian Tuff Carlsen, and Tore Evjen. 2005. Stress responses in Atlantic salmon (Salmo salar L.) smolts during commercial well boat transports, and effects on survival after transfer to sea. Aquaculture 243: 373–382. https://doi.org/10.1016/j.aquaculture.2004.10.019.
79 Hjelle, Bibbi Maria Kállay, Albert Kjartan Dagbjartarson Imsland, Pablo Vigo Balseiro, and Sigurd Olav Handeland. 2022. Acoustic Delicing of Atlantic Salmon (Salmo salar): Fish Welfare and Salmon Lice (Lepeophtheirus salmonis) Dynamics. Journal of Marine Science and Engineering 10: 1004. https://doi.org/10.3390/jmse10081004.
80 EFSA. 2009. Species-specific welfare aspects of the main systems of stunning and killing of farmed Atlantic Salmon. EFSA Journal 7: 1–77. https://doi.org/10.2903/j.efsa.2009.1011.
81 Skjervold, Per Olav, Svein Olav Fjæra, Per Braarød Østby, and Olai Einen. 2001. Live-chilling and crowding stress before slaughter of Atlantic salmon (Salmo salar). Aquaculture 192: 265–280. https://doi.org/10.1016/S0044-8486(00)00447-6.
82 McCormick, S. D, J. M Shrimpton, J. B Carey, M. F O’Dea, K. E Sloan, S Moriyama, and B. Th Björnsson. 1998. Repeated acute stress reduces growth rate of Atlantic salmon parr and alters plasma levels of growth hormone, insulin-like growth factor I and cortisol. Aquaculture 168: 221–235. https://doi.org/10.1016/S0044-8486(98)00351-2.
83 Kittilsen, Silje, Tim Ellis, Joachim Schjolden, Bjarne O. Braastad, and Øyvind Øverli. 2009. Determining stress-responsiveness in family groups of Atlantic salmon (Salmo salar) using non-invasive measures. Aquaculture 298: 146–152. https://doi.org/10.1016/j.aquaculture.2009.10.009.
84 Madaro, Angelico, Rolf E. Olsen, Tore S. Kristiansen, Lars O. E. Ebbesson, Tom O. Nilsen, Gert Flik, and Marnix Gorissen. 2015. Stress in Atlantic salmon: response to unpredictable chronic stress. Journal of Experimental Biology 218: 2538–2550. https://doi.org/10.1242/jeb.120535.
85 Bui, Samantha, Frode Oppedal, Øyvind J. Korsøen, and Tim Dempster. 2013. Modifying Atlantic salmon behaviour with light or feed stimuli may improve parasite control techniques. Aquaculture Environment Interactions 3: 125–133. https://doi.org/10.3354/aei00055.
86 Reimer, T., T. Dempster, F. Warren-Myers, A. J. Jensen, and S. E. Swearer. 2016. High prevalence of vaterite in sagittal otoliths causes hearing impairment in farmed fish. Scientific Reports 6. https://doi.org/10.1038/srep25249.
87 Gjerde, Bjarne, Ma. Josefa R. Pante, and Grete Baeverfjord. 2005. Genetic variation for a vertebral deformity in Atlantic salmon (Salmo salar). Aquaculture 244: 77–87. https://doi.org/10.1016/j.aquaculture.2004.12.002.
88 Witten, P. Eckhard, Alex Obach, Ann Huysseune, and Grete Baeverfjord. 2006. Vertebrae fusion in Atlantic salmon (Salmo salar): Development, aggravation and pathways of containment. Aquaculture 258: 164–172. https://doi.org/10.1016/j.aquaculture.2006.05.005.
89 Robb, D. H. F., S. B. Wotton, J. L. McKinstry, N. K. Sørensen, S. C. Kestin, and N. K. Sørensen. 2000. Commercial slaughter methods used on Atlantic salmon: determination of the onset of brain failure by electroencephalography. Veterinary Record 147: 298–303. https://doi.org/10.1136/vr.147.11.298.
90 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
91 Hendry, K, and D Cragg-Hine. 2003. Ecology of the Atlantic Salmon - IN106. 7. Conserving Natura 2000 Rivers Ecology.
92 Amundsen, Per-Arne, Heidi-Marie Gabler, and Lars Sigvald Riise. 2001. Intraspecific food resource partitioning in Atlantic salmon ( Salmo salar) parr in a subarctic river. Aquatic Living Resources 14: 257–265. https://doi.org/10.1016/S0990-7440(01)01127-5.
93 Orlov, Alexander V., Yuri V. Gerasimov, and Oleg M. Lapshin. 2006. The feeding behaviour of cultured and wild Atlantic salmon, Salmo salar L., in the Louvenga River, Kola Peninsula, Russia. ICES J. Mar. Sci. 63: 1297–1303. https://doi.org/10.1016/j.icesjms.2006.05.004.
94 Bell, J. Gordon, John McEvoy, Douglas R. Tocher, Fiona McGhee, Patrick J. Campbell, and John R. Sargent. 2001. Replacement of Fish Oil with Rapeseed Oil in Diets of Atlantic Salmon (Salmo salar) Affects Tissue Lipid Compositions and Hepatocyte Fatty Acid Metabolism. The Journal of Nutrition 131: 1535–1543.
95 Torstensen, B. E., M. Espe, M. Sanden, I. Stubhaug, R. Waagbø, G. -I. Hemre, R. Fontanillas, et al. 2008. Novel production of Atlantic salmon (Salmo salar) protein based on combined replacement of fish meal and fish oil with plant meal and vegetable oil blends. Aquaculture 285: 193–200. https://doi.org/10.1016/j.aquaculture.2008.08.025.
96 Mood, Alison, Elena Lara, Natasha K. Boyland, and Phil Brooke. 2023. Estimating global numbers of farmed fishes killed for food annually from 1990 to 2019. Animal Welfare 32: e12. https://doi.org/10.1017/awf.2023.4.












Lorem ipsum
Something along the lines of: we were aware of the importance of some topics so that we wanted to include them and collect data but not score them. For WelfareChecks | farm, these topics are "domestication level", "feed replacement", and "commercial relevance". The domestication and commercial relevance aspects allow us to analyse the questions whether increasing rate of domestication or relevance in farming worldwide goes hand in hand with better welfare; the feed replacement rather goes in the direction of added suffering for all those species which end up as feed. For a carnivorous species, to gain 1 kg of meat, you do not just kill this one individual but you have to take into account the meat that it was fed during its life in the form of fish meal and fish oil. In other words, carnivorous species (and to a degree also omnivorous ones) have a larger "fish in:fish out" ratio.
Lorem ipsum
Probably, we updated the profile. Check the version number in the head of the page. For more information on the version, see the FAQ about this. Why do we update profiles? Not just do we want to include new research that has come out, but we are continuously developing the database itself. For example, we changed the structure of entries in criteria or we added explanations for scores in the WelfareCheck | farm. And we are always refining our scoring rules.
The centre of the Overview is an array of criteria covering basic features and behaviours of the species. Each of this information comes from our literature search on the species. If we researched a full Dossier on the species, probably all criteria in the Overview will be covered and thus filled. This was our way to go when we first set up the database.
Because Dossiers are time consuming to research, we switched to focusing on WelfareChecks. These are much shorter profiles covering just 10 criteria we deemed important when it comes to behaviour and welfare in aquaculture (and lately fisheries, too). Also, WelfareChecks contain the assessment of the welfare potential of a species which has become the main feature of the fair-fish database over time. Because WelfareChecks do not cover as many criteria as a Dossier, we don't have the information to fill all blanks in the Overview, as this information is "not investigated by us yet".
Our long-term goal is to go back to researching Dossiers for all species covered in the fair-fish database once we set up WelfareChecks for each of them. If you would like to support us financially with this, please get in touch at ffdb@fair-fish.net
See the question "What does "not investigated by us yet" mean?". In short, if we have not had a look in the literature - or in other words, if we have not investigated a criterion - we cannot know the data. If we have already checked the literature on a criterion and could not find anything, it is "no data found yet". You spotted a "no data found yet" where you know data exists? Get in touch with us at ffdb@fair-fish.net!
Once you have clicked on "show details", the entry for a criterion will unfold and display the summarised information we collected from the scientific literature – complete with the reference(s).
As reference style we chose "Springer Humanities (numeric, brackets)" which presents itself in the database as a number in a grey box. Mouse over the box to see the reference; click on it to jump to the bibliography at the bottom of the page. But what does "[x]-[y]" refer to?
This is the way we mark secondary citations. In this case, we read reference "y", but not reference "x", and cite "x" as mentioned in "y". We try to avoid citing secondary references as best as possible and instead read the original source ourselves. Sometimes we have to resort to citing secondarily, though, when the original source is: a) very old or not (digitally) available for other reasons, b) in a language no one in the team understands. Seldomly, it also happens that we are running out of time on a profile and cannot afford to read the original. As mentioned, though, we try to avoid it, as citing mistakes may always happen (and we don't want to copy the mistake) and as misunderstandings may occur by interpreting the secondarily cited information incorrectly.
If you spot a secondary reference and would like to send us the original work, please contact us at ffdb@fair-fish.net
In general, we aim at giving a good representation of the literature published on the respective species and read as much as we can. We do have a time budget on each profile, though. This is around 80-100 hours for a WelfareCheck and around 300 hours for a Dossier. It might thus be that we simply did not come around to reading the paper.
It is also possible, though, that we did have to make a decision between several papers on the same topic. If there are too many papers on one issue than we manage to read in time, we have to select a sample. On certain topics that currently attract a lot of attention, it might be beneficial to opt for the more recent papers; on other topics, especially in basic research on behaviour in the wild, the older papers might be the go-to source.
And speaking of time: the paper you are missing from the profile might have come out after the profile was published. For the publication date, please check the head of the profile at "cite this profile". We currently update profiles every 6-7 years.
If your paper slipped through the cracks and you would like us to consider it, please get in touch at ffdb@fair-fish.net
This number, for example "C | 2.1 (2022-11-02)", contains 4 parts:
- "C" marks the appearance – the design level – of the profile part. In WelfareChecks | farm, appearance "C" is our most recent one with consistent age class and label (WILD, FARM, LAB) structure across all criteria.
- "2." marks the number of major releases within this appearance. Here, it is major release 2. Major releases include e.g. changes of the WelfareScore. Even if we just add one paper – if it changes the score for one or several criteria, we will mark this as a major update for the profile. With a change to a new appearance, the major release will be re-set to 1.
- ".1" marks the number of minor updates within this appearance. Here, it is minor update 1. With minor updates, we mean changes in formatting, grammar, orthography. It can also mean adding new papers, but if these papers only confirm the score and don't change it, it will be "minor" in our book. With a change to a new appearance, the minor update will be re-set to 0.
- "(2022-11-02)" is the date of the last change – be it the initial release of the part, a minor, or a major update. The nature of the changes you may find out in the changelog next to the version number.
If an Advice, for example, has an initial release date and then just a minor update date due to link corrections, it means that – apart from correcting links – the Advice has not been updated in a major way since its initial release. Please take this into account when consulting any part of the database.
First up, you will find answers to questions for the specific page you are on. Scrolling down in the FAQ window, there are also answers to more general questions. Explore our website and the other sub pages and find there the answers to questions relevant for those pages.
In the fair-fish database, when you have chosen a species (either by searching in the search bar or in the species tree), the landing page is an Overview, introducing the most important information to know about the species that we have come across during our literatures search, including common names, images, distribution, habitat and growth characteristics, swimming aspects, reproduction, social behaviour but also handling details. To dive deeper, visit the Dossier where we collect all available ethological findings (and more) on the most important aspects during the life course, both biologically and concerning the habitat. In contrast to the Overview, we present the findings in more detail citing the scientific references.
Depending on whether the species is farmed or wild caught, you will be interested in different branches of the database.
Farm branch
Founded in 2013, the farm branch of the fair-fish database focuses on farmed aquatic species.
Catch branch
Founded in 2022, the catch branch of the fair-fish database focuses on wild-caught aquatic species.
The heart of the farm branch of the fair-fish database is the welfare assessment – or WelfareCheck | farm – resulting in the WelfareScore | farm for each species. The WelfareCheck | farm is a condensed assessment of the species' likelihood and potential for good welfare in aquaculture, based on welfare-related findings for 10 crucial criteria (home range, depth range, migration, reproduction, aggregation, aggression, substrate, stress, malformations, slaughter).
For those species with a Dossier, we conclude to-be-preferred farming conditions in the Advice | farm. They are not meant to be as detailed as a rearing manual but instead, challenge current farming standards and often take the form of what not to do.
In parallel to farm, the main element of the catch branch of the fair-fish database is the welfare assessment – or WelfareCheck | catch – with the WelfareScore | catch for each species caught with a specific catching method. The WelfareCheck | catch, too, is a condensed assessment of the species' likelihood and potential for good welfare – or better yet avoidance of decrease of good welfare – this time in fisheries. We base this on findings on welfare hazards in 10 steps along the catching process (prospection, setting, catching, emersion, release from gear, bycatch avoidance, sorting, discarding, storing, slaughter).
In contrast to the farm profiles, in the catch branch we assess the welfare separately for each method that the focus species is caught with. In the case of a species exclusively caught with one method, there will be one WelfareCheck, whereas in other species, there will be as many WelfareChecks as there are methods to catch the species with.
Summarising our findings of all WelfareChecks | catch for one species in Advice | catch, we conclude which catching method is the least welfare threatening for this species and which changes to the gear or the catching process will potentially result in improvements of welfare.
Welfare of aquatic species is at the heart of the fair-fish database. In our definition of welfare, we follow Broom (1986): “The welfare of an individual is its state as regards its attempts to cope with its environment.” Thus, welfare may be perceived as a continuum on which an individual rates “good” or “poor” or everything in between.
We pursue what could be called a combination of not only a) valuing the freedom from injuries and stress (function-based approach) but b) supporting attempts to provide rewarding experiences and cognitive challenges (feelings-based approach) as well as c) arguing for enclosures that mimic the wild habitat as best as possible and allow for natural behaviour (nature-based approach).
Try mousing over the element you are interested in - oftentimes you will find explanations this way. If not, there will be FAQ on many of the sub-pages with answers to questions that apply to the respective sub-page. If your question is not among those, contact us at ffdb@fair-fish.net.
It's right here! We decided to re-name it to fair-fish database for several reasons. The database has grown beyond dealing purely with ethology, more towards welfare in general – and so much more. Also, the partners fair-fish and FishEthoGroup decided to re-organise their partnership. While maintaining our friendship, we also desire for greater independence. So, the name "fair-fish database" establishes it as a fair-fish endeavour.