Information
Authors: Jenny Volstorf, Maria Filipa Castanheira
Version: C | 2.1Published: 2024-12-31
- minor editorial changes plus new side note "Commercial relevance"
WelfareScore | farm
The score card gives our welfare assessments for aquatic species in 10 criteria.
For each criterion, we score the probability to experience good welfare under minimal farming conditions ("Likelihood") and under high-standard farming conditions ("Potential") representing the worst and best case scenario. The third dimension scores how certain we are of our assessments based on the number and quality of sources we found ("Certainty").
The WelfareScore sums just the "High" scores in each dimension. Although good welfare ("High") seems not possible in some criteria, there could be at least a potential improvement from low to medium welfare (indicated by ➚ and the number of criteria).
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
➚ = potential improvements not reaching "High" - Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10 per dimension)
General remarks
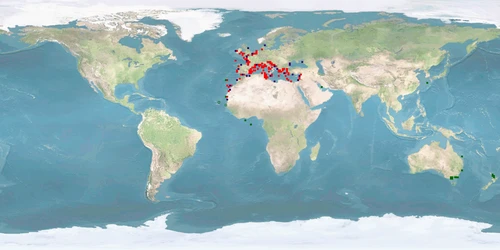
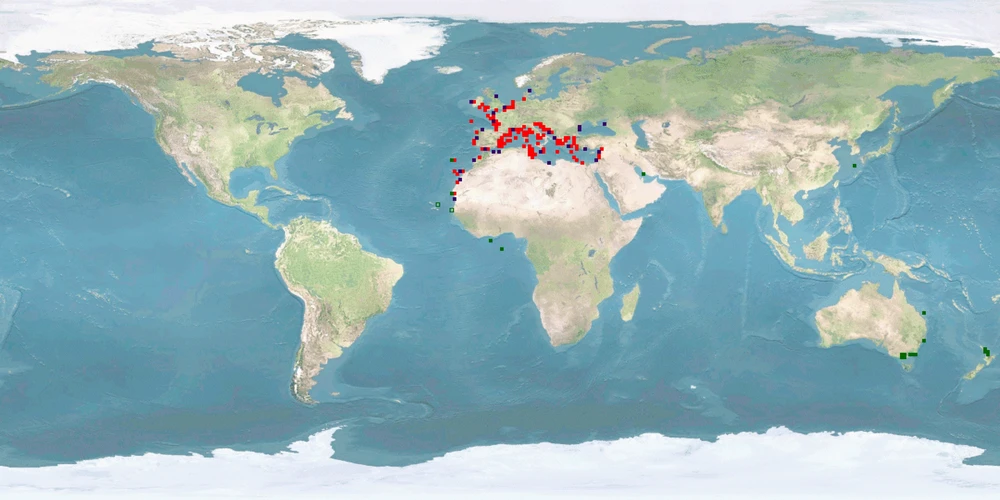
Sparus aurata is a sparid from the eastern Atlantic and the Mediterranean, representing one of the most frequently farmed species in Mediterranean marine finfish aquaculture besides Dicentrarchus labrax. It is mostly cultured in sea cages, to a lesser degree also in tanks, raceways, and ponds. The very low WelfareScore of Sparus aurata is mainly due to high levels of aggression, needs of substrate, stress under farming conditions, and high levels of deformations. Extensive farming providing substrate could be a remediation for some of the problems and help improve fish welfare. Individual farming strategies with mandatory protocols including continuous monitoring are a major stepping stone towards preventing poor welfare and improving the sustainable production of this species. Further research is needed on current farming conditions as well as home range use and aggregation behaviour in the wild.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
There are unclear findings for minimal and high-standard farming conditions, as the natural home range is either partially or completely unknown for all age classes. Our conclusion is based on a low amount of evidence, as the distance moved in the wild comes from one paper only.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions, as the range in captivity at least overlaps with the range in the wild. Our conclusion is based on a medium amount of evidence.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal and high-standard farming conditions, as the species undertakes extensive migrations, and we cannot be sure that providing each age class with their respective environmental conditions will satisfy their urge to migrate or whether they need to experience the transition. Our conclusion is based on a high amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of these circumstances?
It is low for minimal farming conditions. It is medium for high-standard farming conditions, as natural spawning is possible, but omitting of manipulation needs to be verified for the farming context. Our conclusion is based on a low amount of evidence, as knowledge is missing about courtship and whether the species is allowed to perform it as well as whether stripping is applied.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is unclear for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal and high-standard farming conditions, as aggression is present in all age classes, and it is not clear whether size grading and more predictable food dispersal will decrease it. Our conclusion is based on a medium amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions, as cages without substrate prevail. It is medium for high-standard farming conditions, as innovations for enrichment need to be verified for the farming context. Our conclusion is based on a medium amount of evidence.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions. It is medium for high-standard farming conditions, as innovations to reduce stress need to be verified for the focus species or for the farming context. Our conclusion is based on a medium amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is low for minimal farming conditions, as malformation rates exceed 10%. It is medium for high-standard farming conditions, as some malformations result from conditions that may be changed (rearing intensity, forced swimming, inheritance, handling). Our conclusion is based on a medium amount of evidence, as improvement of the situation by adjusting conditions needs more proof.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is low for minimal farming conditions. It is high for high-standard farming conditions, as a) percussive stunning followed by bleeding or b) spiking followed by ice slurry induce unconsciousness fast, kill while still unconscious, and are verified for the farming context. Our conclusion is based on a medium amount of evidence.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 5 83, fully domesticated. Cultured since 1980 11.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes:
- WILD: carnivorous 20 24 84 13.
- FARM: FRY: tanks: live feed Brachionus plicatilis and Artemia 5; ponds: no exogenous feed, zooplankton bloom 5. Fish oil in early weaning diets may be completely replaced by sustainable sources 85. JUVENILES: fish meal may be not 86 or partly* 87 88 or completely* 89 replaced by sustainable sources with concurrent increase in fish oil or partly* replaced without increase in fish oil 90, fish oil may be completely* replaced by sustainable sources 91. SPAWNERS: fish oil may be mostly* replaced by sustainable sources 92.
- LAB: JUVENILES: fish meal may be mostly* replaced by sustainable sources or non-forage fishery components 93, fish oil may be completely* replaced by a mixture of sustainable sources and non-forage fishery components 85.
* partly = <51% – mostly = 51-99% – completely = 100%
Side note: Commercial relevance
How much is this species farmed annually?
258,754 t/year 1990-2019 amounting to estimated 647,000,000-863,000,000 IND/year 1990-2019 94.
Glossary
AMPHIDROMOUS = migrating between fresh water and sea independent of spawning
DOMESTICATION LEVEL 5 = selective breeding programmes are used focusing on specific goals 83
EURYHALINE = tolerant of a wide range of salinities
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FRY = larvae from external feeding on
IND = individuals
JUVENILES = fully developed but immature individuals
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening
PHOTOPERIOD = duration of daylight
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
WILD = setting in the wild
Bibliography
2 Ladino, A., V. Puig-Pons, V. Espinosa, I. Pérez-Arjona, F. de la Gándara, and A. Ortega. 2022. Ultrasonic monitoring of early larval development of fish in tanks. Case study: Gilthead seabream (Sparus aurata). Aquacultural Engineering 98: 102263. https://doi.org/10.1016/j.aquaeng.2022.102263.
3 José, Ricardo Jorge de Freitas. 2012. Sparus aurata larvae production in mesocosm: evaluation of abiotic and biotic parameters. Master, Porto: ICBAS.
4 Özden, Osman, Şahin Saka, and Cüneyt Suzer. 2020. Current status of gilthead seabream (Sparus aurata) and European seabass (Dicentrarchus labrax) production in Turkey. In Marine aquaculture in Turkey: Advancements and management, ed. Deniz Çoban, M. Didem Demircan, and Deniz D. Tosun, 50–68. 59. Istanbul, Turkey: Turkish Marine Research Foundation.
5 Divanach, P., and M. Kentouri. 2000. Hatchery techniques for specific diversification in Mediterranean finfish larviculture. Cahiers options méditerranéennes 47: 75–87.
6 Mercier, Lény, David Mouillot, Olivier Bruguier, Laurent Vigliola, and Audrey M. Darnaude. 2012. Multi-element otolith fingerprints unravel sea−lagoon lifetime migrations of gilthead sea bream Sparus aurata. Marine Ecology Progress Series 444: 175–194. https://doi.org/10.3354/meps09444.
7 Abecasis, David, and Karim Erzini. 2008. Site fidelity and movements of gilthead sea bream (Sparus aurata) in a coastal lagoon (Ria Formosa, Portugal). Estuarine, Coastal and Shelf Science 79: 758–763. https://doi.org/10.1016/j.ecss.2008.06.019.
8 Pousão-Ferreira, Pedro. 2009. Piscicultura em Mar Aberto. Revista de Marinha, May.
9 Papaharisis, Leonidas, Theofania Tsironi, Arkadios Dimitroglou, Petros Taoukis, and Michail Pavlidis. 2019. Stress assessment, quality indicators and shelf life of three aquaculture important marine fish, in relation to harvest practices, water temperature and slaughter method. Aquaculture Research 50: 2608–2620. https://doi.org/10.1111/are.14217.
10 Ortega, Aurelio. 2008. Cultivo de dorada (Sparus aurata). Cuadernos de Acuicultura. Fundación OESA, Observatorio Español de Acuicultura.
11 Colloca, F., and S. Cerasi. 2005. Cultured Aquatic Species Information Programme. Sparus aurata. Rome: FAO Fisheries and Aquaculture Department.
12 Guidetti, P., and S. Bussotti. 2002. Effects of seagrass canopy removal on fish in shallow Mediterranean seagrass (Cymodocea nodosa and Zostera noltii) meadows: a local-scale approach. Marine Biology 140: 445–453. https://doi.org/10.1007/s00227-001-0725-1.
13 Tancioni, L., S. Mariani, A. Maccaroni, A. Mariani, F. Massa, M. Scardi, and S. Cataudella. 2003. Locality-specific variation in the feeding of Sparus aurata L.: evidence from two Mediterranean lagoon systems. Estuarine, Coastal and Shelf Science 57: 469–474. https://doi.org/10.1016/S0272-7714(02)00376-1.
14 Chaoui, Lamya, Mohamed Hichem Kara, and Jean Pierre Quignard. 2006. Growth and reproduction of the gilthead seabream Sparus aurata in Mellah lagoon (north-eastern Algeria). Scientia Marina 70: 545–552. https://doi.org/10.3989/scimar.2006.70n3545.
15 Franco, Anita, Piero Franzoi, Stefano Malavasi, Federico Riccato, Patrizia Torricelli, and Danilo Mainardi. 2006. Use of shallow water habitats by fish assemblages in a Mediterranean coastal lagoon. Estuarine, Coastal and Shelf Science 66: 67–83. https://doi.org/10.1016/j.ecss.2005.07.020.
16 Katselis, George, Katerina Koukou, Evagelos Dimitriou, and Constantin Koutsikopoulos. 2007. Short-term seaward fish migration in the Messolonghi–Etoliko lagoons (Western Greek coast) in relation to climatic variables and the lunar cycle. Estuarine, Coastal and Shelf Science 73: 571–582. https://doi.org/10.1016/j.ecss.2007.02.010.
17 Ahmed, Mohamed S. 2011. Population dynamics and fisheries management of Gilthead sea bream, Sparus aurata (f. Sparidae) from Bardawil lagoon, North Sinai, Egypt. Egypt J. Aquat. Biol. & Fish. 15: 57–69.
18 Balik, Ismet, and Yilmaz Emre. 2013. Monthly variation in stock density and growth performance of juvenile gilthead seabream (Sparus aurata L., 1758) in Beymelek Lagoon, Antalya, Turkey. Pakistan J. Zool. 45: 687–693.
19 Verdiell-Cubedo, David, Francisco J. Oliva-Paterna, Ana Ruiz-Navarro, and Mar Torralva. 2013. Assessing the nursery role for marine fish species in a hypersaline coastal lagoon (Mar Menor, Mediterranean Sea). Marine Biology Research 9: 739–748. https://doi.org/10.1080/17451000.2013.765580.
20 Bauchot, M.-L., J.-C. Hureau, and J. C. Miguel. 1981. Sparidae. In FAO species identification sheets for fishery purposes. Eastern Central Atlantic., ed. W. Fischer, G. Bianchi, and W. B. Scott. Vol. 4. Rome: FAO.
21 Kraljević, Miro, and Jakov Dulčić. 1997. Age and growth of gilt-head sea bream (Sparus aurata L.) in the Mirna estuary, northern Adriatic. Fisheries Research 31: 249–255. https://doi.org/10.1016/S0165-7836(97)00016-7.
22 Alves, Filipe M. A., and Catarina M. A. Alves. 2002. Two new records of seabreams (Pisces:Sparidae) from the Madeira Archipelago.
23 Moretti, Alessandro, Mario Pedini Fernandez-Criado, Giancarlo Cittolin, and Ruggero Guidastri. 1999. Manual on Hatchery Production of Seabass and Gilthead Seabream. Vol. 1. Rome: Food and Agriculture Organization of the United Nations.
24 Ferrari, I., and A. R. Chieregato. 1981. Feeding habits of juvenile stages of Sparus auratus L., Dicentrarchus labrax L. and Mugilidae in a brackish embayment of the Po River Delta. Aquaculture 25: 243–257. https://doi.org/10.1016/0044-8486(81)90186-1.
25 Mariani, Stefano. 2006. Life-history- and ecosystem-driven variation in composition and residence pattern of seabream species (Perciformes: Sparidae) in two Mediterranean coastal lagoons. Marine Pollution Bulletin 53. Recent Developments in Estuarine Ecology and Management: 121–127. https://doi.org/10.1016/j.marpolbul.2005.09.019.
26 Bagni, M., C. Civitareale, A. Priori, A. Ballerini, M. Finoia, G. Brambilla, and G. Marino. 2007. Pre-slaughter crowding stress and killing procedures affecting quality and welfare in sea bass (Dicentrarchus labrax) and sea bream (Sparus aurata). Aquaculture 263: 52–60. https://doi.org/10.1016/j.aquaculture.2006.07.049.
27 Arechavala-Lopez, P., I. Uglem, D. Fernandez-Jover, J. T. Bayle-Sempere, and P. Sanchez-Jerez. 2012. Post-escape dispersion of farmed seabream (Sparus aurata L.) and recaptures by local fisheries in the Western Mediterranean Sea. Fisheries Research 121–122: 126–135. https://doi.org/10.1016/j.fishres.2012.02.003.
28 Brusléa-Sicard, S., and B. Fourcault. 1997. Recognition of sex-inverting protandric Sparus aurata: ultrastructural aspects. Journal of Fish Biology 50: 1094–1103. https://doi.org/10.1111/j.1095-8649.1997.tb01633.x.
29 Mehanna, Sahar Fahmy. 2007. A preliminary assessment and management of gilthead bream Sparus aurata in the Port Said fishery, the Southeastern Mediterranean, Egypt. Turkish Journal of Fisheries and Aquatic Sciences 7: 123–130.
30 Mercier, Lény, Jacques Panfili, Christelle Paillon, Awa N’diaye, David Mouillot, and Audrey M. Darnaude. 2011. Otolith reading and multi-model inference for improved estimation of age and growth in the gilthead seabream Sparus aurata (L.). Estuarine, Coastal and Shelf Science 92: 534–545. https://doi.org/10.1016/j.ecss.2011.02.001.
31 Barbaro, A., A. Francescon, G. Bozzato, A. Merlin, P. Belvedere, and L. Colombo. 1997. Induction of spawning in gilthead seabream, Sparus aurata L., by a long-acting GnRH agonist and its effects on egg quality and daily timing of spawning. Aquaculture 154: 349–359. https://doi.org/10.1016/S0044-8486(97)00067-7.
32 Wong, Ten-Tsao, Shigeho Ijiri, and Yonathan Zohar. 2006. Molecular Biology of Ovarian Aromatase in Sex Reversal: Complementary DNA and 5′-Flanking Region Isolation and Differential Expression of Ovarian Aromatase in the Gilthead Seabream (Sparus aurata). Biology of Reproduction 74: 857–864. https://doi.org/10.1095/biolreprod.105.045351.
33 Arabaci, Muhammed, Yasin Yilmaz, Saltuk Bugrahan Ceyhun, Orhan Erdogan, Hakan Galip Dorlay, Ibrahim Diler, Suleyman Akhan, et al. 2010. A Review on Population Characteristics of Gilthead Seabream (Sparus aurata). Journal of Animal and Veterinary Advances 9: 976–981. https://doi.org/10.3923/javaa.2010.976.981.
34 Jobling, Malcolm, and Stefano Peruzzi. 2010. Seabreams and Porgies (Family: Sparidae). In Finfish Aquaculture Diversification, ed. Nathalie R. Le Francois, Malcolm Jobling, Chris Carter, and Pierre Blier, 361–373. CABI.
35 Sarà, G., A. Oliveri, G. Martino, S. Serra, G. Meloni, and A. Pais. 2010. Response of captive seabass and seabream as behavioural indicator in aquaculture. Italian Journal of Animal Science 6: 823–825. https://doi.org/10.4081/ijas.2007.1s.823.
36 Arechavala-Lopez, Pablo, Joan Nazzaro-Alvarez, Andrea Jardí-Pons, Lourdes Reig, Francesca Carella, Maite Carrassón, and Ana Roque. 2020. Linking stocking densities and feeding strategies with social and individual stress responses on gilthead seabream (Sparus aurata). Physiology & Behavior 213: 112723. https://doi.org/10.1016/j.physbeh.2019.112723.
37 Tort, L., J. O. Sunyer, E. Gómez, and A. Molinero. 1996. Crowding stress induces changes in serum haemolytic and agglutinating activity in the gilthead sea bream Sparus aurata. Veterinary Immunology and Immunopathology 51: 179–188. https://doi.org/10.1016/0165-2427(95)05502-9.
38 Montero, D., M. S. Izquierdo, L. Tort, L. Robaina, and J. M. Vergara. 1999. High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata, juveniles. Fish Physiology and Biochemistry 20: 53–60. https://doi.org/10.1023/A:1007719928905.
39 Carbonara, Pierluigi, Sebastien Alfonso, Walter Zupa, Amedeo Manfrin, Eleonora Fiocchi, Tobia Pretto, Maria Teresa Spedicato, and Giuseppe Lembo. 2019. Behavioral and physiological responses to stocking density in sea bream (Sparus aurata): Do coping styles matter? Physiology & Behavior 212: 112698. https://doi.org/10.1016/j.physbeh.2019.112698.
40 Tandler, A., M. Har’el, M. Wilks, A. Levinson, L. Brickell, S. Christie, E. Avital, and Y. Barr. 1989. Effect of environmental temperature on survival, growth and population structure in the mass rearing of the gilthead seabream, Sparus aurata. Aquaculture 78: 277–284. https://doi.org/10.1016/0044-8486(89)90105-1.
41 Arechavala-Lopez, P., C. Diaz-Gil, J. L. Saraiva, D. Moranta, M. F. Castanheira, S. Nuñez-Velázquez, S. Ledesma-Corvi, M. R. Mora-Ruiz, and A. Grau. 2019. Effects of structural environmental enrichment on welfare of juvenile seabream (Sparus aurata). Aquaculture Reports 15: 100224. https://doi.org/10.1016/j.aqrep.2019.100224.
42 Cammarata, M., M. Vazzana, D. Accardi, and N. Parrinello. 2012. Seabream (Sparus aurata) long-term dominant-subordinate interplay affects phagocytosis by peritoneal cavity cells. Brain, Behavior, and Immunity 26: 580–587. https://doi.org/10.1016/j.bbi.2012.01.008.
43 Castanheira, Maria Filipa, Marcelino Herrera, Benjamín Costas, Luís E. C. Conceição, and Catarina I. M. Martins. 2013. Linking cortisol responsiveness and aggressive behaviour in gilthead seabream Sparus aurata: Indication of divergent coping styles. Applied Animal Behaviour Science 143: 75–81. https://doi.org/10.1016/j.applanim.2012.11.008.
44 Goldan, Oded, Dan Popper, and IIan Karplus. 2003. Food Competition In Small Groups Of Juvenile Gilthead Sea Bream (Sparus Aurata). The Israeli Journal of Aquaculture - Bamidgeh 55: 94–106.
45 Montero, D., G. Lalumera, M. S. Izquierdo, M. J. Caballero, M. Saroglia, and L. Tort. 2009. Establishment of dominance relationships in gilthead sea bream Sparus aurata juveniles during feeding: effects on feeding behaviour, feed utilization and fish health. Journal of Fish Biology 74: 790–805. https://doi.org/10.1111/j.1095-8649.2008.02161.x.
46 Oikonomidou, Eleni, Alkisti Batzina, and Nafsika Karakatsouli. 2019. Effects of food quantity and distribution on aggressive behaviour of gilthead seabream and European seabass. Applied Animal Behaviour Science 213: 124–130. https://doi.org/10.1016/j.applanim.2019.02.010.
47 Andrew, J. E, J Holm, S Kadri, and F. A Huntingford. 2004. The effect of competition on the feeding efficiency and feed handling behaviour in gilthead sea bream (Sparus aurata L.) held in tanks. Aquaculture 232: 317–331. https://doi.org/10.1016/S0044-8486(03)00528-3.
48 Batzina, Alkisti, and Nafsika Karakatsouli. 2012. The presence of substrate as a means of environmental enrichment in intensively reared gilthead seabream Sparus aurata: Growth and behavioral effects. Aquaculture 370–371: 54–60. https://doi.org/10.1016/j.aquaculture.2012.10.005.
49 Batzina, Alkisti, Christina Dalla, Zeta Papadopoulou-Daifoti, and Nafsika Karakatsouli. 2014. Effects of environmental enrichment on growth, aggressive behaviour and brain monoamines of gilthead seabream Sparus aurata reared under different social conditions. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 169: 25–32. https://doi.org/10.1016/j.cbpa.2013.12.001.
50 Batzina, Alkisti, Dimitris Kalogiannis, Christina Dalla, Zeta Papadopoulou-Daifoti, Stella Chadio, and Nafsika Karakatsouli. 2014. Blue substrate modifies the time course of stress response in gilthead seabream Sparus aurata. Aquaculture 420–421: 247–253. https://doi.org/10.1016/j.aquaculture.2013.11.016.
51 Batzina, Alkisti, Christina Dalla, Aristeidis Tsopelakos, Zeta Papadopoulou-Daifoti, and Nafsika Karakatsouli. 2014. Environmental enrichment induces changes in brain monoamine levels in gilthead seabream Sparus aurata. Physiology & Behavior 130: 85–90. https://doi.org/10.1016/j.physbeh.2014.03.023.
52 Reyes-Tomassini, Jose J. 2009. Behavioral and Neuroendocrine Correlates of Sex Change in the Gilthead Seabream (Sparus aurata). University of Maryland.
53 Sánchez-Lamadrid, A. 2004. Effectiveness of releasing gilthead sea bream (Sparus aurata, L.) for stock enhancement in the bay of Cádiz. Aquaculture 231: 135–148. https://doi.org/10.1016/j.aquaculture.2003.08.015.
54 Craig, G., D. Paynter, I. Coscia, and S. Mariani. 2008. Settlement of gilthead sea bream Sparus aurata L. in a southern Irish Sea coastal habitat. Journal of Fish Biology 72: 287–291. https://doi.org/10.1111/j.1095-8649.2007.01644.x.
55 Katselis, George, Constantin Koutsikopoulos, Evagelos Dimitriou, and Yiannis Rogdakis. 2003. Spatial patterns and temporal trends in the fisheries landings of the Messolonghi-Etoliko lagoons (Western Greek Coast). Scientia Marina 67. https://doi.org/10.3989/scimar.2003.67n4501.
56 Batzina, Alkisti, Kyriaki Sotirakoglou, and Nafsika Karakatsouli. 2014. The preference of 0+ and 2+ gilthead seabream Sparus aurata for coloured substrates or no-substrate. Applied Animal Behaviour Science 151: 110–116. https://doi.org/10.1016/j.applanim.2013.11.013.
57 Arechavala-Lopez, P., J. C. Caballero-Froilán, M. Jiménez-García, X. Capó, S. Tejada, J. L. Saraiva, A. Sureda, and D. Moranta. 2020. Enriched environments enhance cognition, exploratory behaviour and brain physiological functions of Sparus aurata. Scientific Reports 10: 11252. https://doi.org/10.1038/s41598-020-68306-6.
58 Koven, W., Y. Barr, S. Lutzky, I. Ben-Atia, R. Weiss, M. Harel, P. Behrens, and A. Tandler. 2001. The effect of dietary arachidonic acid (20:4n−6) on growth, survival and resistance to handling stress in gilthead seabream (Sparus aurata) larvae. Aquaculture 193: 107–122. https://doi.org/10.1016/S0044-8486(00)00479-8.
59 Alves Martins, Dulce, Filipa Rocha, Gonzalo Martínez-Rodríguez, Gordon Bell, Sofia Morais, Filipa Castanheira, Narcisa Bandarra, Joana Coutinho, Manuel Yúfera, and Luís E. C. Conceição. 2012. Teleost fish larvae adapt to dietary arachidonic acid supply through modulation of the expression of lipid metabolism and stress response genes. The British Journal of Nutrition 108: 864–874. https://doi.org/10.1017/S0007114511006143.
60 Van Anholt, R. D., W. M. Koven, S. Lutzky, and S. E. Wendelaar Bonga. 2004. Dietary supplementation with arachidonic acid alters the stress response of gilthead seabream (Sparus aurata) larvae. Aquaculture 238: 369–383. https://doi.org/10.1016/j.aquaculture.2004.06.001.
61 Orduna, Carlos, Lourdes Encina, Amadora Rodríguez-Ruiz, and Victoria Rodríguez-Sánchez. 2021. Testing of new sampling methods and estimation of size structure of sea bass (Dicentrarchus labrax) in aquaculture farms using horizontal hydroacoustics. Aquaculture 545: 737242. https://doi.org/10.1016/j.aquaculture.2021.737242.
62 Rotllant, J., P. H. M. Balm, J. Pérez-Sánchez, S. E. Wendelaar-Bonga, and L. Tort. 2001. Pituitary and Interrenal Function in Gilthead Sea Bream (Sparus aurata L., Teleostei) after Handling and Confinement Stress. General and Comparative Endocrinology 121: 333–342. https://doi.org/10.1006/gcen.2001.7604.
63 Arends, R. J., J. M. Mancera, J. L. Munoz, SE Wendelaar Bonga, and G. Flik. 1999. The stress response of the gilthead sea bream (Sparus aurata L.) to air exposure and confinement. Journal of Endocrinology 163: 149–157. https://doi.org/10.1677/joe.0.1630149.
64 Ortuño, J., M. A. Esteban, and J. Meseguer. 2001. Effects of short-term crowding stress on the gilthead seabream (Sparus aurata L.) innate immune response. Fish & Shellfish Immunology 11: 187–197. https://doi.org/10.1006/fsim.2000.0304.
65 Filiciotto, Francesco, Vincenzo Maximiliano Giacalone, Francesco Fazio, Gaspare Buffa, Giuseppe Piccione, Vincenzo Maccarrone, Vincenzo Di Stefano, Salvatore Mazzola, and Giuseppa Buscaino. 2013. Effect of acoustic environment on gilthead sea bream (Sparus aurata): Sea and onshore aquaculture background noise. Aquaculture 414–415: 36–45. https://doi.org/10.1016/j.aquaculture.2013.07.042.
66 Matos, Elisabete, Amparo Gonçalves, Maria Leonor Nunes, Maria Teresa Dinis, and Jorge Dias. 2010. Effect of harvesting stress and slaughter conditions on selected flesh quality criteria of gilthead seabream (Sparus aurata). Aquaculture 305: 66–72. https://doi.org/10.1016/j.aquaculture.2010.04.020.
67 Boglione, Clara, Flavio Gagliardi, Michele Scardi, and Stefano Cataudella. 2001. Skeletal descriptors and quality assessment in larvae and post-larvae of wild-caught and hatchery-reared gilthead sea bream (Sparus aurata L. 1758). Aquaculture 192: 1–22. https://doi.org/10.1016/S0044-8486(00)00446-4.
68 Paperna, I. 1978. Swimbladder and skeletal deformations in hatchery bred Spams aurata. Journal of Fish Biology 12: 109–114. https://doi.org/10.1111/j.1095-8649.1978.tb04157.x.
69 Andrades, J. A., J. Becerra, and P. Fernández-Llebrez. 1996. Skeletal deformities in larval, juvenile and adult stages of cultured gilthead sea bream (Sparus aurata L.). Aquaculture 141: 1–11. https://doi.org/10.1016/0044-8486(95)01226-5.
70 Koumoundouros, G., G. Oran, P. Divanach, S. Stefanakis, and M. Kentouri. 1997. The opercular complex deformity in intensive gilthead sea bream (Sparus aurata L.) larviculture. Moment of apparition and description. Aquaculture 156: 165–177. https://doi.org/10.1016/S0044-8486(97)89294-0.
71 Castro, Jaime, Ania Pino-Querido, Miguel Hermida, David Chavarrías, Roberto Romero, Luis A. García-Cortés, Miguel A. Toro, and Paulino Martínez. 2008. Heritability of skeleton abnormalities (lordosis, lack of operculum) in gilthead seabream (Sparus aurata) supported by microsatellite family data. Aquaculture 279: 18–22. https://doi.org/10.1016/j.aquaculture.2008.04.023.
72 Chatain, Beatrice. 1994. Abnormal swimbladder development and lordosis in sea bass (Dicentrarchus labrax) and sea bream (Sparus auratus). Aquaculture 119: 371–379. https://doi.org/0044-8486/94.
73 Carrillo, J, G Koumoundouros, P Divanach, and J Martinez. 2001. Morphological malformations of the lateral line in reared gilthead sea bream (Sparus aurata L. 1758). Aquaculture 192: 281–290. https://doi.org/10.1016/S0044-8486(00)00454-3.
74 Sfakianakis, D. G., P. Katharios, N. Tsirigotakis, C. K. Doxa, and M. Kentouri. 2013. Lateral line deformities in wild and farmed sea bass (Dicentrarchus labrax, L.) and sea bream (Sparus aurata, L.). Journal of Applied Ichthyology 29: 1015–1021. https://doi.org/10.1111/jai.12248.
75 Negrín-Báez, D., A. Navarro, I. Lee-Montero, M. Soula, J. M. Afonso, and M. J. Zamorano. 2015. Inheritance of skeletal deformities in gilthead seabream (Sparus aurata) - lack of operculum, lordosis, vertebral fusion and LSK complex. Journal of Animal Science 93: 53–61. https://doi.org/10.2527/jas.2014-7968.
76 Balebona, M. C., M. A. Morinigo, J. A. Andrades, J. A. Santamaria, J. Becerra, and J. S. Borrego. 1993. Microbiological study of gilthead sea bream S. aurata L. affected by lordosis a skeletal deformity. Bull. Eur. Assoc. Fish Pathol. 13: 33.
77 Afonso, J. M., D. Montero, L. Robaina, N. Astorga, M. S. Izquierdo, and R. Ginés. 2000. Association of a lordosis-scoliosis-kyphosis deformity in gilthead seabream (Sparus aurata) with family structure. Fish Physiology and Biochemistry 22: 159–163. https://doi.org/10.1023/A:1007811702624.
78 EFSA. 2009. Species-specific welfare aspects of the main systems of stunning and killing of farmed Seabass and Seabream. EFSA Journal 1010: 1–52. https://doi.org/10.2903/j.efsa.2009.1010.
79 Saraiva, João L. 2018. Personal communication.
80 de la Rosa, Ignacio, Pedro L. Castro, and Rafael Ginés. 2021. Twenty Years of Research in Seabass and Seabream Welfare during Slaughter. Animals 11: 2164. https://doi.org/10.3390/ani11082164.
81 van De Vis, Hans, Steve Kestin, David Robb, Jörg Oehlenschläger, Bert Lambooij, Werner Münkner, Holmer Kuhlmann, et al. 2003. Is humane slaughter of fish possible for industry? Aquaculture Research 34: 211–220. https://doi.org/10.1046/j.1365-2109.2003.00804.x.
82 Roque, A., N. Gras, S. Rey-Planellas, E. Fatsini, J. Pallisera, N. Duncan, I. Muñoz, A. Velarde, and M. D. Hernandez. 2021. The feasibility of using gas mixture to stun seabream (Sparus aurata) before slaughtering in aquaculture production. Aquaculture 545: 737168. https://doi.org/10.1016/j.aquaculture.2021.737168.
83 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
84 Pita, C., S. Gamito, and K. Erzini. 2002. Feeding habits of the gilthead seabream (Sparus aurata) from the Ria Formosa (southern Portugal) as compared to the black seabream (Spondyliosoma cantharus) and the annular seabream (Diplodus annularis). Journal of Applied Ichthyology 18: 81–86. https://doi.org/10.1046/j.1439-0426.2002.00336.x.
85 Carvalho, Marta, Bruno Marotta, Hanlin Xu, Pierre-André Geraert, Sachi Kaushik, Daniel Montero, and Marisol Izquierdo. 2022. Complete replacement of fish oil by three microalgal products rich in n-3 long-chain polyunsaturated fatty acids in early weaning microdiets for gilthead sea bream (Sparus aurata). Aquaculture 558: 738354. https://doi.org/10.1016/j.aquaculture.2022.738354.
86 Sitjà-Bobadilla, A., S. Peña-Llopis, P. Gómez-Requeni, F. Médale, S. Kaushik, and J. Pérez-Sánchez. 2005. Effect of fish meal replacement by plant protein sources on non-specific defence mechanisms and oxidative stress in gilthead sea bream (Sparus aurata). Aquaculture 249: 387–400. https://doi.org/10.1016/j.aquaculture.2005.03.031.
87 Robaina, L., M. S. Izquierdo, F. J. Moyano, J. Socorro, J. M. Vergara, D. Montero, and H. Fernández-Palacios. 1995. Soybean and lupin seed meals as protein sources in diets for gilthead seabream (Sparus aurata): nutritional and histological implications. Aquaculture 130: 219–233. https://doi.org/10.1016/0044-8486(94)00225-D.
88 Kissil, G Wm, I Lupatsch, D A Higgs, and R W Hardy. 2000. Dietary substitution of soy and rapeseed protein concentrates for fish meal, and their effects on growth and nutrient utilization in gilthead seabream Sparus aurata L. Aquaculture Research 31: 595–601. https://doi.org/10.1046/j.1365-2109.2000.00477.x.
89 Kissil, George Wm, and Ingrid Lupatsch. 2004. Successful Replacement Of Fishmeal By Plant Proteins In Diets For The Gilthead Seabream, Sparus Aurata L. Israeli Journal of Aquaculture - Bamidgeh 56: 188–199.
90 Savonitto, Gilda, Roy Barkan, Sheenan Harpaz, Amir Neori, Helena Chernova, Antonio Terlizzi, and Lior Guttman. 2021. Fishmeal replacement by periphyton reduces the fish in fish out ratio and alimentation cost in gilthead sea bream Sparus aurata. Scientific Reports 11: 20990. https://doi.org/10.1038/s41598-021-00466-5.
91 Santigosa, Ester, Fabio Brambilla, and Luca Milanese. 2021. Microalgae Oil as an Effective Alternative Source of EPA and DHA for Gilthead Seabream (Sparus aurata) Aquaculture. Animals 11: 971. https://doi.org/10.3390/ani11040971.
92 Izquierdo, M. S., S. Turkmen, D. Montero, M. J. Zamorano, J. M. Afonso, V. Karalazos, and H. Fernández-Palacios. 2015. Nutritional programming through broodstock diets to improve utilization of very low fishmeal and fish oil diets in gilthead sea bream. Aquaculture 449. Proceedings of the 16th International Symposium on Fish Nutrition and Feeding: 18–26. https://doi.org/10.1016/j.aquaculture.2015.03.032.
93 Aragão, Cláudia, Miguel Cabano, Rita Colen, Juan Fuentes, and Jorge Dias. 2020. Alternative formulations for gilthead seabream diets: Towards a more sustainable production. Aquaculture Nutrition 26: 444–455. https://doi.org/10.1111/anu.13007.
94 Mood, Alison, Elena Lara, Natasha K. Boyland, and Phil Brooke. 2023. Estimating global numbers of farmed fishes killed for food annually from 1990 to 2019. Animal Welfare 32: e12. https://doi.org/10.1017/awf.2023.4.










Lorem ipsum
Something along the lines of: we were aware of the importance of some topics so that we wanted to include them and collect data but not score them. For WelfareChecks | farm, these topics are "domestication level", "feed replacement", and "commercial relevance". The domestication and commercial relevance aspects allow us to analyse the questions whether increasing rate of domestication or relevance in farming worldwide goes hand in hand with better welfare; the feed replacement rather goes in the direction of added suffering for all those species which end up as feed. For a carnivorous species, to gain 1 kg of meat, you do not just kill this one individual but you have to take into account the meat that it was fed during its life in the form of fish meal and fish oil. In other words, carnivorous species (and to a degree also omnivorous ones) have a larger "fish in:fish out" ratio.
Lorem ipsum
Probably, we updated the profile. Check the version number in the head of the page. For more information on the version, see the FAQ about this. Why do we update profiles? Not just do we want to include new research that has come out, but we are continuously developing the database itself. For example, we changed the structure of entries in criteria or we added explanations for scores in the WelfareCheck | farm. And we are always refining our scoring rules.
The centre of the Overview is an array of criteria covering basic features and behaviours of the species. Each of this information comes from our literature search on the species. If we researched a full Dossier on the species, probably all criteria in the Overview will be covered and thus filled. This was our way to go when we first set up the database.
Because Dossiers are time consuming to research, we switched to focusing on WelfareChecks. These are much shorter profiles covering just 10 criteria we deemed important when it comes to behaviour and welfare in aquaculture (and lately fisheries, too). Also, WelfareChecks contain the assessment of the welfare potential of a species which has become the main feature of the fair-fish database over time. Because WelfareChecks do not cover as many criteria as a Dossier, we don't have the information to fill all blanks in the Overview, as this information is "not investigated by us yet".
Our long-term goal is to go back to researching Dossiers for all species covered in the fair-fish database once we set up WelfareChecks for each of them. If you would like to support us financially with this, please get in touch at ffdb@fair-fish.net
See the question "What does "not investigated by us yet" mean?". In short, if we have not had a look in the literature - or in other words, if we have not investigated a criterion - we cannot know the data. If we have already checked the literature on a criterion and could not find anything, it is "no data found yet". You spotted a "no data found yet" where you know data exists? Get in touch with us at ffdb@fair-fish.net!
Once you have clicked on "show details", the entry for a criterion will unfold and display the summarised information we collected from the scientific literature – complete with the reference(s).
As reference style we chose "Springer Humanities (numeric, brackets)" which presents itself in the database as a number in a grey box. Mouse over the box to see the reference; click on it to jump to the bibliography at the bottom of the page. But what does "[x]-[y]" refer to?
This is the way we mark secondary citations. In this case, we read reference "y", but not reference "x", and cite "x" as mentioned in "y". We try to avoid citing secondary references as best as possible and instead read the original source ourselves. Sometimes we have to resort to citing secondarily, though, when the original source is: a) very old or not (digitally) available for other reasons, b) in a language no one in the team understands. Seldomly, it also happens that we are running out of time on a profile and cannot afford to read the original. As mentioned, though, we try to avoid it, as citing mistakes may always happen (and we don't want to copy the mistake) and as misunderstandings may occur by interpreting the secondarily cited information incorrectly.
If you spot a secondary reference and would like to send us the original work, please contact us at ffdb@fair-fish.net
In general, we aim at giving a good representation of the literature published on the respective species and read as much as we can. We do have a time budget on each profile, though. This is around 80-100 hours for a WelfareCheck and around 300 hours for a Dossier. It might thus be that we simply did not come around to reading the paper.
It is also possible, though, that we did have to make a decision between several papers on the same topic. If there are too many papers on one issue than we manage to read in time, we have to select a sample. On certain topics that currently attract a lot of attention, it might be beneficial to opt for the more recent papers; on other topics, especially in basic research on behaviour in the wild, the older papers might be the go-to source.
And speaking of time: the paper you are missing from the profile might have come out after the profile was published. For the publication date, please check the head of the profile at "cite this profile". We currently update profiles every 6-7 years.
If your paper slipped through the cracks and you would like us to consider it, please get in touch at ffdb@fair-fish.net
This number, for example "C | 2.1 (2022-11-02)", contains 4 parts:
- "C" marks the appearance – the design level – of the profile part. In WelfareChecks | farm, appearance "C" is our most recent one with consistent age class and label (WILD, FARM, LAB) structure across all criteria.
- "2." marks the number of major releases within this appearance. Here, it is major release 2. Major releases include e.g. changes of the WelfareScore. Even if we just add one paper – if it changes the score for one or several criteria, we will mark this as a major update for the profile. With a change to a new appearance, the major release will be re-set to 1.
- ".1" marks the number of minor updates within this appearance. Here, it is minor update 1. With minor updates, we mean changes in formatting, grammar, orthography. It can also mean adding new papers, but if these papers only confirm the score and don't change it, it will be "minor" in our book. With a change to a new appearance, the minor update will be re-set to 0.
- "(2022-11-02)" is the date of the last change – be it the initial release of the part, a minor, or a major update. The nature of the changes you may find out in the changelog next to the version number.
If an Advice, for example, has an initial release date and then just a minor update date due to link corrections, it means that – apart from correcting links – the Advice has not been updated in a major way since its initial release. Please take this into account when consulting any part of the database.
First up, you will find answers to questions for the specific page you are on. Scrolling down in the FAQ window, there are also answers to more general questions. Explore our website and the other sub pages and find there the answers to questions relevant for those pages.
In the fair-fish database, when you have chosen a species (either by searching in the search bar or in the species tree), the landing page is an Overview, introducing the most important information to know about the species that we have come across during our literatures search, including common names, images, distribution, habitat and growth characteristics, swimming aspects, reproduction, social behaviour but also handling details. To dive deeper, visit the Dossier where we collect all available ethological findings (and more) on the most important aspects during the life course, both biologically and concerning the habitat. In contrast to the Overview, we present the findings in more detail citing the scientific references.
Depending on whether the species is farmed or wild caught, you will be interested in different branches of the database.
Farm branch
Founded in 2013, the farm branch of the fair-fish database focuses on farmed aquatic species.
Catch branch
Founded in 2022, the catch branch of the fair-fish database focuses on wild-caught aquatic species.
The heart of the farm branch of the fair-fish database is the welfare assessment – or WelfareCheck | farm – resulting in the WelfareScore | farm for each species. The WelfareCheck | farm is a condensed assessment of the species' likelihood and potential for good welfare in aquaculture, based on welfare-related findings for 10 crucial criteria (home range, depth range, migration, reproduction, aggregation, aggression, substrate, stress, malformations, slaughter).
For those species with a Dossier, we conclude to-be-preferred farming conditions in the Advice | farm. They are not meant to be as detailed as a rearing manual but instead, challenge current farming standards and often take the form of what not to do.
In parallel to farm, the main element of the catch branch of the fair-fish database is the welfare assessment – or WelfareCheck | catch – with the WelfareScore | catch for each species caught with a specific catching method. The WelfareCheck | catch, too, is a condensed assessment of the species' likelihood and potential for good welfare – or better yet avoidance of decrease of good welfare – this time in fisheries. We base this on findings on welfare hazards in 10 steps along the catching process (prospection, setting, catching, emersion, release from gear, bycatch avoidance, sorting, discarding, storing, slaughter).
In contrast to the farm profiles, in the catch branch we assess the welfare separately for each method that the focus species is caught with. In the case of a species exclusively caught with one method, there will be one WelfareCheck, whereas in other species, there will be as many WelfareChecks as there are methods to catch the species with.
Summarising our findings of all WelfareChecks | catch for one species in Advice | catch, we conclude which catching method is the least welfare threatening for this species and which changes to the gear or the catching process will potentially result in improvements of welfare.
Welfare of aquatic species is at the heart of the fair-fish database. In our definition of welfare, we follow Broom (1986): “The welfare of an individual is its state as regards its attempts to cope with its environment.” Thus, welfare may be perceived as a continuum on which an individual rates “good” or “poor” or everything in between.
We pursue what could be called a combination of not only a) valuing the freedom from injuries and stress (function-based approach) but b) supporting attempts to provide rewarding experiences and cognitive challenges (feelings-based approach) as well as c) arguing for enclosures that mimic the wild habitat as best as possible and allow for natural behaviour (nature-based approach).
Try mousing over the element you are interested in - oftentimes you will find explanations this way. If not, there will be FAQ on many of the sub-pages with answers to questions that apply to the respective sub-page. If your question is not among those, contact us at ffdb@fair-fish.net.
It's right here! We decided to re-name it to fair-fish database for several reasons. The database has grown beyond dealing purely with ethology, more towards welfare in general – and so much more. Also, the partners fair-fish and FishEthoGroup decided to re-organise their partnership. While maintaining our friendship, we also desire for greater independence. So, the name "fair-fish database" establishes it as a fair-fish endeavour.