Information
Authors: Jenny Volstorf, Paolo Panizzon, João L. Saraiva
Version: C | 1.0Published: 2025-07-08
- profile update resulting in major editorial and content changes (changing the scoring in criteria 1-8, 10)
- transfer to consistent age class and label structure resulting in changed appearance
- minor editorial changes plus new side note "Commercial relevance"
WelfareScore | farm
The score card gives our welfare assessments for aquatic species in 10 criteria.
For each criterion, we score the probability to experience good welfare under minimal farming conditions ("Likelihood") and under high-standard farming conditions ("Potential") representing the worst and best case scenario. The third dimension scores how certain we are of our assessments based on the number and quality of sources we found ("Certainty").
The WelfareScore sums just the "High" scores in each dimension. Although good welfare ("High") seems not possible in some criteria, there could be at least a potential improvement from low to medium welfare (indicated by ➚ and the number of criteria).
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
➚ = potential improvements not reaching "High" - Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10 per dimension)
General remarks
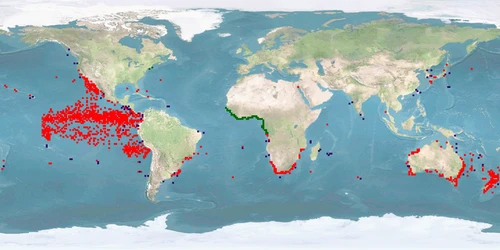
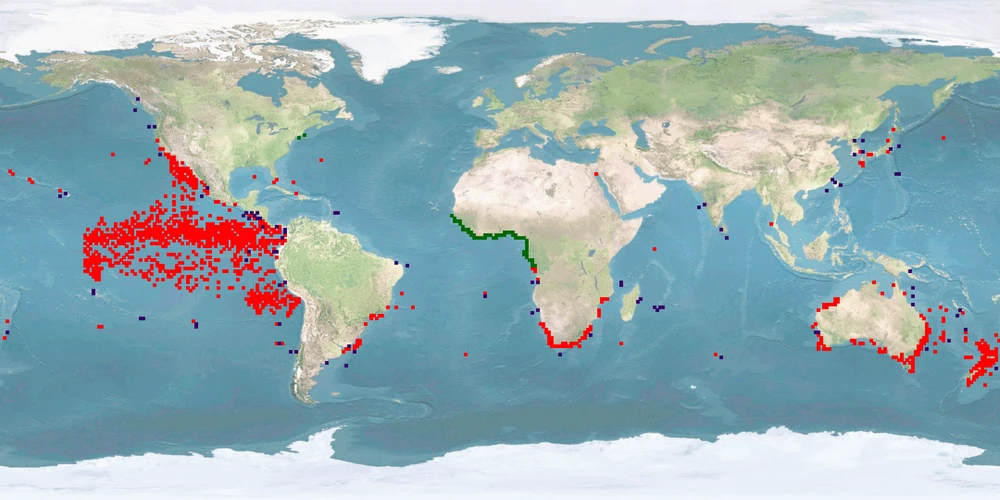
Seriola lalandi is a member of the family Carangidae. It is a PELAGIC, gregarious species that can be found in temperate and subtropical waters of the Southern Hemisphere. While being a popular target for recreational fisheries, it is not considered at conservation risk. Commercial farming is well-established in Australia and expanding to New Zealand, Chile, Peru, and Northern Europe. Reasons for this development are the fast growth of up to 3 kg per year and easy reproduction – although currently, some farmers still rely on wild-caught breeders. Rearing consists of a hatchery stage until FINGERLINGS size and then transfer to tanks or sea cages where the IND are grown out for 12 up to 32 months.
Despite growing interest in S. lalandi for aquaculture, its ecology during the early life stages and its migratory patterns are not fully understood. Having a carnivorous diet means an unsustainable feed in farms and makes a complete replacement with feed alternatives impossible.
The WelfareScore is low due to higher spatial needs than captivity is able to provide, manipulations during reproduction, high stocking densities, aggression, missing substrate, handling stress, and high malformation rates. Non-assisted reproduction in captivity is possible. We need further knowledge whether high densities are compatible with a schooling species, keeping in mind that ADULTS in the wild can be solitary. Although S. lalandi is considered PELAGIC, it has been found over reefs or at fish-aggregating devices. Further research should show whether substrate in farms can be beneficial. A protocol for humane killing is available and applied – we need further knowledge whether it is the standard worldwide.
Note: the status of Seriola lalandi as a species has been revised after a series of papers investigated the genetic relatedness of different populations 1 2 3. Because of that, older sources are not fully reliable regarding the identification of S. lalandi in the northern hemisphere, especially in the Pacific 1. For example, we skipped papers on "California yellowtail", even if they were referred to as Seriola lalandi, as this species is now generally referred to as Seriola dorsalis1 4. Also, we skipped wild papers on (allegedly) S. lalandi when they came from the Northern hemisphere.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
It is low for minimal farming conditions, as tanks and cages do not cover the higher end of the home range in the wild, although we cannot be sure in some age classes. It is unclear for high-standard farming conditions, as we lack details on the lower end of the home range in the wild. Our conclusion is based on a medium amount of evidence, as further research is needed on specific home range information in the wild.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal farming conditions, as tanks and cages do not cover the higher end of the depth range in the wild, although we cannot be sure in some age classes. It is medium for high-standard farming conditions, as the mentioned systems at least overlap with the range in the wild. Our conclusion is based on a high amount of evidence unless farm studies show that S. lalandi is well under lower depths as in the wild.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal farming conditions, as the species undertakes more or less extensive migrations, and we cannot be sure that providing each age class with their respective environmental conditions will satisfy their urge to migrate or whether they need to experience the transition. It is unclear for high-standard farming conditions, as we lack details on the lower end of the migration range. Our conclusion is based on a medium amount of evidence, as further research is needed on specific migration distances in the wild.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of these circumstances?
It is low for minimal farming conditions, as the species is manipulated (PHOTOPERIOD+temperature or hormonal manipulation) and may be taken from the wild. It is high for high-standard farming conditions, as natural breeding (without manipulation) with farm-reared IND is possible and verified for the farming context (albeit not in combination). Our conclusion is based on a medium amount of evidence, as further research is needed on reproduction behaviour in the wild, and we would like to see the combination of non-assisted reproduction with farm-reared IND applied in farms.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is low for minimal farming conditions, as densities in some tanks go beyond the smallest density in the wild (although hard to compare given different units in WILD and FARM). It is medium for high-standard farming conditions given the schooling tendency of the species that might translate to an overlap of densities in some tanks and sea cages with the density range in the wild. Our conclusion is based on a medium amount of evidence, as further research is needed on densities in the wild.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal and high-standard farming conditions, as the species is aggressive – even cannibalistic – in some age classes and as there are no promising ways to reduce aggression besides sufficient feeding – and size grading which is potentially stressful itself and needs to be verified for the farming context. Our conclusion is based on a medium amount of evidence, as further research is needed.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions, as JUVENILES to SPAWNERS are found close to substrate (although unclear whether they need it), but tanks and cages are devoid of it. It is unclear for high-standard farming conditions, as we lack studies investigating whether JUVENILES to SPAWNERS are well without substrate or could benefit from it. Our conclusion is based on a medium amount of evidence, as further research is needed on the specified issue.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions, as the species is stressed (water quality, handling, size grading [potentially], transport). It is medium for high-standard farming conditions, as one way to reduce (but not avoid) stress is verified for the farming context (Pro-Tex). Our conclusion is based on a medium amount of evidence, as other mitigation measures need to be verified for the farming context.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is low for minimal farming conditions, as malformation rates may exceed 10%. It is medium for high-standard farming conditions, as some malformations result from conditions that may be changed (water quality, spawning time, feed, aeration). Our conclusion is based on a medium amount of evidence, as improvement of the situation by adjusting conditions needs more proof.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is unclear for minimal farming conditions, as it was difficult to get an overview of which stunning methods are applied. It is high for high-standard farming conditions, as ikejime (if it includes a stunning step) induces unconsciousness fast, kills while still unconscious, and is verified for the farming context. Our conclusion is based on a medium amount of evidence, as further research is needed.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 3 30, level 5 being fully domesticated.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes:
- WILD: carnivorous 26 25.
- FARM: FRY are fed with rotifers 11. Fish meal may be mostly* replaced by a combination of fish and poultry by-products or partly* by sustainable sources 59 60. Potential of replacing fish oil mostly* by poultry oil 61, but further research needed for effects on growth.
- LAB: for JUVENILES, fish meal may not be replaced by certain sustainable sources 62, but partly* replaced by other sustainable sources 48 63 while increasing fish oil 64 65, and mostly* replaced by non-forage fishery components 66. For JUVENILES, fish oil may be completely* replaced by poultry 67 and partly* replaced by canola oil 67, although further research is needed to determine the cause of green liver.
*partly = <51%, mostly = 51-99%, completely = 100%
Side note: Commercial relevance
How much is this species farmed annually?
407 t/year 1990-2019 amounting to estimated <1,000,000 IND/year 1990-2019 68.
Glossary
DOMESTICATION LEVEL 3 = entire life cycle closed in captivity with wild inputs 58
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FINGERLINGS = early juveniles with fully developed scales and working fins, the size of a human finger
FRY = larvae from external feeding on
IND = individuals
JUVENILES = fully developed but immature individuals
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening
OCEANODROMOUS = living and migrating in the sea
PELAGIC = living independent of bottom and shore of a body of water
PHOTOPERIOD = duration of daylight
PLANKTONIC = horizontal movement limited to hydrodynamic displacement
RAS = Recirculating Aquaculture System - almost completely closed system using filters to clean and recirculate water with the aim of reducing water input and with the advantage of enabling close control of environmental parameters to maintain high water quality
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
TOTAL LENGTH = from snout to tip of caudal fin as compared to fork length (which measures from snout to fork of caudal fin) or standard length (from head to base of tail fin) or body length (from the base of the eye notch to the posterior end of the telson) 43
WILD = setting in the wild
Bibliography
2 Swart, Bl, Ae Bester-van Der Merwe, Se Kerwath, and R Roodt-Wilding. 2016. Phylogeography of the pelagic fish Seriola lalandi at different scales: confirmation of inter-ocean population structure and evaluation of southern African genetic diversity. African Journal of Marine Science 38: 513–524. https://doi.org/10.2989/1814232X.2016.1238410.
3 Premachandra, H. K. A., Fabiola Lafarga-De La Cruz, Yutaka Takeuchi, Adam Miller, Stewart Fielder, Wayne O’Connor, Celine H. Frère, Nguyen Hong Nguyen, Ido Bar, and Wayne Knibb. 2017. Genomic DNA variation confirmed Seriola lalandi comprises three different populations in the Pacific, but with recent divergence. Scientific Reports 7: 9386. https://doi.org/10.1038/s41598-017-07419-x.
4 ITIS Team. 2025. Seriola dorsalis (Gill, 1863). ITIS Integrated Taxonomic Information System. https://itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=168696&print_version=SCR&source=from_print#null. Accessed July 2.
5 Sumida, B. Y., H. G. Moser, and E. H. Ahlstrom. 1985. Descriptions of larvae of California yellowtail, Seriola lalandi, and three other carangids from the eastern tropical Pacific: Chloroscombrus orqueta, Caranx caballus, and Caranx sexfasciatus. Calif. Coop. Ocean. Fish. Investig. Rep 26: 139–159.
6 Cobcroft, Jennifer M., Patricia M. Pankhurst, Carolyn Poortenaar, Bob Hickman, and Mike Tait. 2004. Jaw malformation in cultured yellowtail kingfish (Seriola lalandi) larvae. New Zealand Journal of Marine and Freshwater Research 38: 67–71. https://doi.org/10.1080/00288330.2004.9517218.
7 Toledo, Cesar, Eduardo Rubilar, Lorena Marchant, Jessica Dörner, Lorenzo Márquez, Víctor Martínez, and Patricio Dantagnan. 2023. Relationship between Jaw Malformations and Long-Chain PUFA’s in Seriola lalandi Larvae during the Spawning Season at a Commercial Hatchery. Fishes 8: 200. https://doi.org/10.3390/fishes8040200.
8 Hilton, Zoë, Carolyn W. Poortenaar, and Mary A. Sewell. 2008. Lipid and protein utilisation during early development of yellowtail kingfish (Seriola lalandi). Marine Biology 154: 855–865. https://doi.org/10.1007/s00227-008-0978-z.
9 Moran, Damian. 2007. Size heterogeneity, growth potential and aggression in juvenile yellowtail kingfish (Seriola lalandi Valenciennes). Aquaculture Research 38: 1254–1264. https://doi.org/10.1111/j.1365-2109.2007.01769.x.
10 Nguyen, N H, P Whatmore, A Miller, and W Knibb. 2016. Quantitative genetic properties of four measures of deformity in yellowtail kingfish Seriola lalandi Valenciennes, 1833. Journal of Fish Diseases 39: 217–228. https://doi.org/10.1111/jfd.12348.
11 Fielder, D. Stewart. 2013. Hatchery production of yellowtail kingfish ( Seriola lalandi ). In Advances in Aquaculture Hatchery Technology, 542–553. Elsevier. https://doi.org/10.1533/9780857097460.3.542.
12 Tanner, Jason E., and Milena Fernandes. 2010. Environmental effects of yellowtail kingfish aquaculture in South Australia. Aquacult Environ Interact 1: 155–165.
13 Clarke, Thomas M., Sasha K. Whitmarsh, Fabrice R. A. Jaine, Matt D. Taylor, Stephanie Brodie, Nicholas L. Payne, Paul A. Butcher, Matt K. Broadhurst, Joshua Davey, and Charlie Huveneers. 2024. Environmental drivers of yellowtail kingfish, Seriola lalandi, activity inferred through a continental acoustic tracking network. Aquatic Conservation: Marine and Freshwater Ecosystems 34: e4019. https://doi.org/10.1002/aqc.4019.
14 Hutson, K. S., B. P. Smith, R. T. Godfrey, I. D. Whittington, C. B. Chambers, I. Ernst, and B. M. Gillanders. 2007. A Tagging Study on Yellowtail Kingfish (Seriola Lalandi) and Samson Fish (S. Hippos) in South Australian Waters. Transactions of the Royal Society of South Australia 131: 128–134. https://doi.org/10.1080/03721426.2007.10887075.
15 Goddard, Belinda K., Tristan A. Guillemin, Hayden T. Schilling, Julian M. Hughes, Stephanie Brodie, Corey P. Green, Robert Harcourt, et al. 2024. Half a century of citizen science tag-recapture data reveals stock delineation and cross-jurisdictional connectivity of an iconic pelagic fish. Reviews in Fish Biology and Fisheries 34: 1433–1449. https://doi.org/10.1007/s11160-024-09880-0.
16 Gillanders, Bronwyn M., Douglas J. Ferrell, and Neil L. Andrew. 2001. Estimates of movement and life-history parameters of yellowtail kingfish (Seriola lalandi): how useful are data from a cooperative tagging programme? Marine and Freshwater Research 52: 179–192. https://doi.org/10.1071/MF99153.
17 Holdsworth, J.C., and P.J. Saul. 2014. New Zealand Billfish and Gamefish Tagging, 2012–13.
18 Park, Ju Yeong. 2022. Investigating the use of light to manipulate the vertical distribution of yellowtail kingfish (Seriola lalandi) in recirculating aquaculture systems (RAS). Master, Auckland, Australia: University of Auckland.
19 Government of South Australia. 2023. Zoning In: South Australian Aquaculture Report.
20 Moran, Damian, Cea K. Smith, Brendan Gara, and Carolyn W. Poortenaar. 2007. Reproductive behaviour and early development in yellowtail kingfish (Seriola lalandi Valenciennes 1833). Aquaculture 262: 95–104. https://doi.org/10.1016/j.aquaculture.2006.10.005.
21 Martínez-Porchas, Marcel, Fabiola Lafarga-De la Cruz, Felipe Aguilera, Francesco Cicala, and Asunción Lago-Lestón. 2021. Water microbiota is not affected by stocking density of the yellowtail kingfish (Seriola lalandi) in a recirculating aquaculture system. Aquaculture Research 52: 410–414. https://doi.org/10.1111/are.14883.
22 Moran, D, Ck Smith, Ps Lee, and Sj Pether. 2011. Mortality structures population size characteristics of juvenile yellowtail kingfish Seriola lalandi reared at different densities. Aquatic Biology 11: 229–238. https://doi.org/10.3354/ab00314.
23 Dempster, T. 2005. Temporal variability of pelagic fish assemblages around fish aggregation devices: biological and physical influences. Journal of Fish Biology 66: 1237–1260. https://doi.org/10.1111/j.0022-1112.2005.00674.x.
24 Gillanders, B. M., D. J. Ferrell, and N. L. Andrew. 1999. Size at maturity and seasonal changes in gonad activity of yellowtail kingfish (Seriola lalandi; Carangidae) in New South Wales, Australia. New Zealand Journal of Marine and Freshwater Research 33: 457–468. https://doi.org/10.1080/00288330.1999.9516891.
25 Dunn, K. 2014. The diet, reproductive biology, age and growth of yellowtail, Seriola lalandi. Master, Cape Town: University of Cape Town.
26 Vergani, M., E.M. Acha, J.M. Diaz De Astarloa, and D. Giberto. 2008. Food of the yellowtail amberjack Seriola lalandi from the south-west Atlantic. Journal of the Marine Biological Association of the United Kingdom 88: 851–852. https://doi.org/10.1017/S0025315408000477.
27 Riede, K. 2004. Global register of migratory species - from global to regional scales. Final report of the R&D Projekt 808 05 081. Bonn, Germany: Federal Agency for Nature Conservation.
28 Kerwath, Sven, Rouvay Roodt-Wilding, Toufiek Samaai, Henning Winker, Wendy West, Sheroma Surajnarayan, Belinda Swart, et al. 2021. Shallow seamounts represent speciation islands for circumglobal yellowtail Seriola lalandi. Scientific Reports 11: 3559. https://doi.org/10.1038/s41598-021-82501-z.
29 Volstorf, Jenny. 2025. Conclusion.
30 Panizzon, Paolo. 2025. Conclusion.
31 Becker, Alistair, Michael B Lowry, D Stewart Fielder, and Matthew D Taylor. 2021. Dispersal of yellowtail kingfish (Seriola lalandi) from a coastal embayment following a recreational fisheries enhancement stocking program: attempts to integrate aquaculture and habitat-based initiatives. Bulletin of Marine Science 97: 615–630. https://doi.org/10.5343/bms.2021.0013.
32 Silvano, R. A. M., P. F. L. MacCord, R. V. Lima, and A. Begossi. 2006. When Does this Fish Spawn? Fishermen’s Local Knowledge of Migration and Reproduction of Brazilian Coastal Fishes. Environmental Biology of Fishes 76: 371–386. https://doi.org/10.1007/s10641-006-9043-2.
33 Penney, A. 1982. The southern Cape yellowtail fishery - a research perspective and preliminary results. 108. South Africa: Sea Fisheries Research Insitute.
34 Garratt, P. A. 1988. Notes on seasonal abundance and spawning of some important offshore linefish in Natal and Transkei waters, southern Africa. South African Journal of Marine Science 7: 1–8. https://doi.org/10.2989/025776188784379161.
35 Poortenaar, C.W., S.H. Hooker, and N. Sharp. 2001. Assessment of yellowtail kingfish (Seriola lalandi lalandi) reproductive physiology, as a basis for aquaculture development. Aquaculture 201: 271–286. https://doi.org/10.1016/S0044-8486(01)00549-X.
36 Penney, A. J. Personal communication.
37 Ma, Zhenhua, Daniel Aik Yang Tan, and Jian G Qin. 2014. Jaw deformities in the larvae of yellowtail kingfish (Seriola lalandi Valenciennes, 1833) from two groups of broodstock. Indian Journal of Fisheries 61.
38 Booth, Mark A, Luke Cheviot, Brendan Findlay, Wayne Knibb, and David S Fielder. 2019. Impact of changing the diet of Yellowtail Kingfish (Seriola lalandi) broodstock on fecundity, egg quality and heredity. In Growing a profitable, innovative and collaborative australian Yellowtail Kingfish aquaculture industry, ed. David AJ Stone, Mark A. Booth, and Steven M. Clarke, 449–472. Fisheries Research and Development Corporation; South Australian Research and Development Institute; New South WalesDpeartment of Primary Industries.
39 Dettleff, P., E. Hernandez, Gavin Partridge, Fabiola Lafarga-De la Cruz, and V. Martinez. 2020. Understanding the population structure and reproductive behavior of hatchery-produced yellowtail kingfish (Seriola lalandi). Aquaculture 522: 734948. https://doi.org/10.1016/j.aquaculture.2020.734948.
40 Setiawan, A. N., S. Muncaster, S. Pether, A. King, G. W. Irvine, P. M. Lokman, and J. E. Symonds. 2016. The effects of gonadotropin-releasing hormone analog on yellowtail kingfish Seriola lalandi (Valenciennes, 1833) spawning and egg quality. Aquaculture Reports 4: 1–9. https://doi.org/10.1016/j.aqrep.2016.05.001.
41 Heagney, Ec, Tp Lynch, Rc Babcock, and Im Suthers. 2007. Pelagic fish assemblages assessed using mid-water baited video: standardising fish counts using bait plume size. Marine Ecology Progress Series 350: 255–266. https://doi.org/10.3354/meps07193.
42 Na-Nakorn, Uthairat, Manoch Kamcharoen, Brian S. Santos, Shenna Kate M. Torres, Masamishi Nakajima, Wansuk Senanan, Maria Mojena Gonzales-Plasus, and Cong Zeng. 2023. Current status of carangid aquaculture and way forward. Agriculture and Natural Resources 57: 541–558. https://doi.org/10.34044/j.anres.2023.57.3.18.
43 Pawson, M.G., and G.D. Pickett. 1996. The Annual Pattern of Condition and Maturity in Bass, Dicentrarchus Labrax, in Waters Around England and Wales. Journal of the Marine Biological Association of the United Kingdom 76: 107. https://doi.org/10.1017/S0025315400029040.
44 Carton, Alexander G. 2005. The impact of light intensity and algal-induced turbidity on first-feeding Seriola lalandi larvae. Aquaculture Research 36: 1588–1594. https://doi.org/10.1111/j.1365-2109.2005.01383.x.
45 Boerrigter, Jeroen G. J., Hans W. van de Vis, Ruud van den Bos, Wout Abbink, Tom Spanings, Jan Zethof, Laura Louzao Martinez, Wouter F. M. van Andel, Javier Lopez-Luna, and Gert Flik. 2014. Effects of Pro-Tex on zebrafish (Danio rerio) larvae, adult common carp (Cyprinus carpio) and adult yellowtail kingfish (Seriola lalandi). Fish Physiology and Biochemistry 40: 1201–1212. https://doi.org/10.1007/s10695-014-9916-9.
46 Moran, Damian, Rufus M G Wells, and Stephen J Pether. 2008. Low stress response exhibited by juvenile yellowtail kingfish (Seriola lalandi Valenciennes) exposed to hypercapnic conditions associated with transportation. Aquaculture Research 39: 1399–1407. https://doi.org/10.1111/j.1365-2109.2008.02009.x.
47 Woolley, Lindsey D., Luke W. Pilmer, Frances J. Stephens, Zi X. Lim, Peter G. Arthur, Hosna GholipourKanani, and Gavin J. Partridge. 2022. The effect of hydrogen peroxide concentration and water temperature on yellowtail kingfish Seriola lalandi in a repeated bathing treatment. Aquaculture 560: 738545. https://doi.org/10.1016/j.aquaculture.2022.738545.
48 Booth, M.A., M.D. Moses, and G.L. Allan. 2013. Utilisation of carbohydrate by yellowtail kingfish Seriola lalandi. Aquaculture 376–379: 151–161. https://doi.org/10.1016/j.aquaculture.2012.11.024.
49 Abbink, Wout, Ainhoa Blanco Garcia, Jonathan A.C. Roques, Gavin J. Partridge, Kees Kloet, and Oliver Schneider. 2012. The effect of temperature and pH on the growth and physiological response of juvenile yellowtail kingfish Seriola lalandi in recirculating aquaculture systems. Aquaculture 330–333: 130–135. https://doi.org/10.1016/j.aquaculture.2011.11.043.
50 Stone, David A J, Shane D Roberts, and Krishna-Lee Currie. 2014. Hyper-Saline Conditions Affect Growth, Osmoregulation and Survival of Fingerling and Juvenile Yellowtail Kingfish, Seriola Lalandi. Journal of Aquaculture & Marine Biology 1: 00005. https://doi.org/10.15406/jamb.2014.01.00005.
51 Cobcroft, Jennifer M., and Stephen C. Battaglene. 2013. Skeletal malformations in Australian marine finfish hatcheries. Aquaculture 396–399: 51–58. https://doi.org/10.1016/j.aquaculture.2013.02.027.
52 Fielder, D. Stewart. 2013. Unpublished data.
53 Cobcroft, Jennifer M., Stephen C. Battaglene, J.C.G. Biggs, and David S. Fielder. 2013. Investigating causes of skeletal malformation in yellowtail kingfish Seriola lalandi. In , ed. C. I. Hendry, 81–84. Ghent University, Belgium.
54 Iki jime or ike jime (pronounced “iki jimi”) is a humane method of killing fish. 2025. Ikijime. https://www.ikijime.com/iki-jime-humane-killing-of-fish/. Accessed July 7.
55 The Kingfish company. 2025. Methodology. The Kingfish Company.
56 Drønen, Ole Andreas. 2024. Switch to kingfish is going swimmingly in Fredrikstad. August 8.
57 Llonch, P., E. Lambooij, H.G.M. Reimert, and J.W. van de Vis. 2012. Assessing effectiveness of electrical stunning and chilling in ice water of farmed yellowtail kingfish, common sole and pike-perch. Aquaculture 364–365: 143–149. https://doi.org/10.1016/j.aquaculture.2012.08.015.
58 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
59 Bansemer, Matthew S., Michael J. Salini, Leo Nankervis, and David A. J. Stone. 2023. Reducing dietary wild derived fishmeal inclusion levels in production diets for large yellowtail kingfish (Seriola lalandi). Aquaculture 572: 739487. https://doi.org/10.1016/j.aquaculture.2023.739487.
60 Crowe, Benjamin H., James O. Harris, Todd J. McWhorter, Matthew S. Bansemer, and David A. J. Stone. 2024. Liver Structure and Function in Yellowtail Kingfish, Seriola lalandi, in Response to Reduced Fish Meal Diets. Aquaculture, Fish and Fisheries 4: e70026. https://doi.org/10.1002/aff2.70026.
61 Crowe, Benjamin H., James O. Harris, Todd J. McWhorter, Matthew S. Bansemer, and David A. J. Stone. 2025. Liver structure and function in yellowtail kingfish, Seriola lalandi, in response to alternative oils in feed. Aquaculture 594: 741379. https://doi.org/10.1016/j.aquaculture.2024.741379.
62 Bowyer, Jenna N., Jian G. Qin, Richard P. Smullen, Louise R. Adams, Michael J.S. Thomson, and David A.J. Stone. 2013. The use of a soy product in juvenile yellowtail kingfish (Seriola lalandi) feeds at different water temperatures: 1. Solvent extracted soybean meal. Aquaculture 384–387: 35–45. https://doi.org/10.1016/j.aquaculture.2012.12.005.
63 Pilmer, Luke, Lindsey Woolley, Alan Lymbery, Chinh Dam, Abigail Elizur, Md Javed Foysal, and Gavin Partridge. 2025. Exploring single cell microbial protein as a sustainable fishmeal alternative in yellowtail kingfish (Seriola lalandi) diets: impacts on health and gut microbiome. Journal of Animal Science and Biotechnology 16: 16. https://doi.org/10.1186/s40104-024-01146-w.
64 Bowyer, Jenna N., Jian G. Qin, Richard P. Smullen, Louise R. Adams, Michael J.S. Thomson, and David A.J. Stone. 2013. The use of a soy product in juvenile yellowtail kingfish (Seriola lalandi) feeds at different water temperatures: 2. Soy protein concentrate. Aquaculture 410–411: 1–10. https://doi.org/10.1016/j.aquaculture.2013.06.001.
65 Bansemer, M.S., R.E.A. Forder, G.S. Howarth, G.M. Suitor, J. Bowyer, and D.A.J. Stone. 2015. The effect of dietary soybean meal and soy protein concentrate on the intestinal mucus layer and development of subacute enteritis in Yellowtail Kingfish (Seriola lalandi) at suboptimal water temperature. Aquaculture Nutrition 21: 300–310. https://doi.org/10.1111/anu.12160.
66 Pilmer, Luke, Lindsey Woolley, Alan J. Lymbery, Michael Salini, Chinh Dam, Md Javed Foysal, and Gavin Partridge. 2025. Sustainable Fishmeal Alternatives: Impact of Partially Defatted Black Soldier Fly (Hermetia Illucens) Meal on Growth and Health of Yellowtail Kingfish (Seriola Lalandi). SSRN Scholarly Paper. Rochester, NY: Social Science Research Network. https://doi.org/10.2139/ssrn.5101732.
67 Bowyer, J.N., J.G. Qin, R.P. Smullen, and D.A.J. Stone. 2012. Replacement of fish oil by poultry oil and canola oil in yellowtail kingfish (Seriola lalandi) at optimal and suboptimal temperatures. Aquaculture 356–357: 211–222. https://doi.org/10.1016/j.aquaculture.2012.05.014.
68 Mood, Alison, Elena Lara, Natasha K. Boyland, and Phil Brooke. 2023. Estimating global numbers of farmed fishes killed for food annually from 1990 to 2019. Animal Welfare 32: e12. https://doi.org/10.1017/awf.2023.4.








Lorem ipsum
Something along the lines of: we were aware of the importance of some topics so that we wanted to include them and collect data but not score them. For WelfareChecks | farm, these topics are "domestication level", "feed replacement", and "commercial relevance". The domestication and commercial relevance aspects allow us to analyse the questions whether increasing rate of domestication or relevance in farming worldwide goes hand in hand with better welfare; the feed replacement rather goes in the direction of added suffering for all those species which end up as feed. For a carnivorous species, to gain 1 kg of meat, you do not just kill this one individual but you have to take into account the meat that it was fed during its life in the form of fish meal and fish oil. In other words, carnivorous species (and to a degree also omnivorous ones) have a larger "fish in:fish out" ratio.
Lorem ipsum
Probably, we updated the profile. Check the version number in the head of the page. For more information on the version, see the FAQ about this. Why do we update profiles? Not just do we want to include new research that has come out, but we are continuously developing the database itself. For example, we changed the structure of entries in criteria or we added explanations for scores in the WelfareCheck | farm. And we are always refining our scoring rules.
The centre of the Overview is an array of criteria covering basic features and behaviours of the species. Each of this information comes from our literature search on the species. If we researched a full Dossier on the species, probably all criteria in the Overview will be covered and thus filled. This was our way to go when we first set up the database.
Because Dossiers are time consuming to research, we switched to focusing on WelfareChecks. These are much shorter profiles covering just 10 criteria we deemed important when it comes to behaviour and welfare in aquaculture (and lately fisheries, too). Also, WelfareChecks contain the assessment of the welfare potential of a species which has become the main feature of the fair-fish database over time. Because WelfareChecks do not cover as many criteria as a Dossier, we don't have the information to fill all blanks in the Overview, as this information is "not investigated by us yet".
Our long-term goal is to go back to researching Dossiers for all species covered in the fair-fish database once we set up WelfareChecks for each of them. If you would like to support us financially with this, please get in touch at ffdb@fair-fish.net
See the question "What does "not investigated by us yet" mean?". In short, if we have not had a look in the literature - or in other words, if we have not investigated a criterion - we cannot know the data. If we have already checked the literature on a criterion and could not find anything, it is "no data found yet". You spotted a "no data found yet" where you know data exists? Get in touch with us at ffdb@fair-fish.net!
Once you have clicked on "show details", the entry for a criterion will unfold and display the summarised information we collected from the scientific literature – complete with the reference(s).
As reference style we chose "Springer Humanities (numeric, brackets)" which presents itself in the database as a number in a grey box. Mouse over the box to see the reference; click on it to jump to the bibliography at the bottom of the page. But what does "[x]-[y]" refer to?
This is the way we mark secondary citations. In this case, we read reference "y", but not reference "x", and cite "x" as mentioned in "y". We try to avoid citing secondary references as best as possible and instead read the original source ourselves. Sometimes we have to resort to citing secondarily, though, when the original source is: a) very old or not (digitally) available for other reasons, b) in a language no one in the team understands. Seldomly, it also happens that we are running out of time on a profile and cannot afford to read the original. As mentioned, though, we try to avoid it, as citing mistakes may always happen (and we don't want to copy the mistake) and as misunderstandings may occur by interpreting the secondarily cited information incorrectly.
If you spot a secondary reference and would like to send us the original work, please contact us at ffdb@fair-fish.net
In general, we aim at giving a good representation of the literature published on the respective species and read as much as we can. We do have a time budget on each profile, though. This is around 80-100 hours for a WelfareCheck and around 300 hours for a Dossier. It might thus be that we simply did not come around to reading the paper.
It is also possible, though, that we did have to make a decision between several papers on the same topic. If there are too many papers on one issue than we manage to read in time, we have to select a sample. On certain topics that currently attract a lot of attention, it might be beneficial to opt for the more recent papers; on other topics, especially in basic research on behaviour in the wild, the older papers might be the go-to source.
And speaking of time: the paper you are missing from the profile might have come out after the profile was published. For the publication date, please check the head of the profile at "cite this profile". We currently update profiles every 6-7 years.
If your paper slipped through the cracks and you would like us to consider it, please get in touch at ffdb@fair-fish.net
This number, for example "C | 2.1 (2022-11-02)", contains 4 parts:
- "C" marks the appearance – the design level – of the profile part. In WelfareChecks | farm, appearance "C" is our most recent one with consistent age class and label (WILD, FARM, LAB) structure across all criteria.
- "2." marks the number of major releases within this appearance. Here, it is major release 2. Major releases include e.g. changes of the WelfareScore. Even if we just add one paper – if it changes the score for one or several criteria, we will mark this as a major update for the profile. With a change to a new appearance, the major release will be re-set to 1.
- ".1" marks the number of minor updates within this appearance. Here, it is minor update 1. With minor updates, we mean changes in formatting, grammar, orthography. It can also mean adding new papers, but if these papers only confirm the score and don't change it, it will be "minor" in our book. With a change to a new appearance, the minor update will be re-set to 0.
- "(2022-11-02)" is the date of the last change – be it the initial release of the part, a minor, or a major update. The nature of the changes you may find out in the changelog next to the version number.
If an Advice, for example, has an initial release date and then just a minor update date due to link corrections, it means that – apart from correcting links – the Advice has not been updated in a major way since its initial release. Please take this into account when consulting any part of the database.
First up, you will find answers to questions for the specific page you are on. Scrolling down in the FAQ window, there are also answers to more general questions. Explore our website and the other sub pages and find there the answers to questions relevant for those pages.
In the fair-fish database, when you have chosen a species (either by searching in the search bar or in the species tree), the landing page is an Overview, introducing the most important information to know about the species that we have come across during our literatures search, including common names, images, distribution, habitat and growth characteristics, swimming aspects, reproduction, social behaviour but also handling details. To dive deeper, visit the Dossier where we collect all available ethological findings (and more) on the most important aspects during the life course, both biologically and concerning the habitat. In contrast to the Overview, we present the findings in more detail citing the scientific references.
Depending on whether the species is farmed or wild caught, you will be interested in different branches of the database.
Farm branch
Founded in 2013, the farm branch of the fair-fish database focuses on farmed aquatic species.
Catch branch
Founded in 2022, the catch branch of the fair-fish database focuses on wild-caught aquatic species.
The heart of the farm branch of the fair-fish database is the welfare assessment – or WelfareCheck | farm – resulting in the WelfareScore | farm for each species. The WelfareCheck | farm is a condensed assessment of the species' likelihood and potential for good welfare in aquaculture, based on welfare-related findings for 10 crucial criteria (home range, depth range, migration, reproduction, aggregation, aggression, substrate, stress, malformations, slaughter).
For those species with a Dossier, we conclude to-be-preferred farming conditions in the Advice | farm. They are not meant to be as detailed as a rearing manual but instead, challenge current farming standards and often take the form of what not to do.
In parallel to farm, the main element of the catch branch of the fair-fish database is the welfare assessment – or WelfareCheck | catch – with the WelfareScore | catch for each species caught with a specific catching method. The WelfareCheck | catch, too, is a condensed assessment of the species' likelihood and potential for good welfare – or better yet avoidance of decrease of good welfare – this time in fisheries. We base this on findings on welfare hazards in 10 steps along the catching process (prospection, setting, catching, emersion, release from gear, bycatch avoidance, sorting, discarding, storing, slaughter).
In contrast to the farm profiles, in the catch branch we assess the welfare separately for each method that the focus species is caught with. In the case of a species exclusively caught with one method, there will be one WelfareCheck, whereas in other species, there will be as many WelfareChecks as there are methods to catch the species with.
Summarising our findings of all WelfareChecks | catch for one species in Advice | catch, we conclude which catching method is the least welfare threatening for this species and which changes to the gear or the catching process will potentially result in improvements of welfare.
Welfare of aquatic species is at the heart of the fair-fish database. In our definition of welfare, we follow Broom (1986): “The welfare of an individual is its state as regards its attempts to cope with its environment.” Thus, welfare may be perceived as a continuum on which an individual rates “good” or “poor” or everything in between.
We pursue what could be called a combination of not only a) valuing the freedom from injuries and stress (function-based approach) but b) supporting attempts to provide rewarding experiences and cognitive challenges (feelings-based approach) as well as c) arguing for enclosures that mimic the wild habitat as best as possible and allow for natural behaviour (nature-based approach).
Try mousing over the element you are interested in - oftentimes you will find explanations this way. If not, there will be FAQ on many of the sub-pages with answers to questions that apply to the respective sub-page. If your question is not among those, contact us at ffdb@fair-fish.net.
It's right here! We decided to re-name it to fair-fish database for several reasons. The database has grown beyond dealing purely with ethology, more towards welfare in general – and so much more. Also, the partners fair-fish and FishEthoGroup decided to re-organise their partnership. While maintaining our friendship, we also desire for greater independence. So, the name "fair-fish database" establishes it as a fair-fish endeavour.