Information
Author: Pablo Arechavala-Lopez
Version: B | 1.2Published: 2024-12-31
- minor editorial changes plus new side note "Commercial relevance"
WelfareScore | farm
The score card gives our welfare assessments for aquatic species in 10 criteria.
For each criterion, we score the probability to experience good welfare under minimal farming conditions ("Likelihood") and under high-standard farming conditions ("Potential") representing the worst and best case scenario. The third dimension scores how certain we are of our assessments based on the number and quality of sources we found ("Certainty").
The WelfareScore sums just the "High" scores in each dimension. Although good welfare ("High") seems not possible in some criteria, there could be at least a potential improvement from low to medium welfare (indicated by ➚ and the number of criteria).
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
➚ = potential improvements not reaching "High" - Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10 per dimension)
General remarks
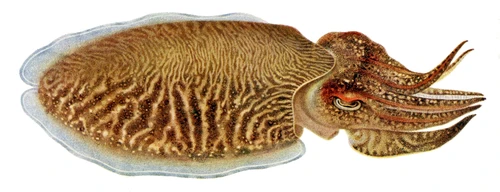
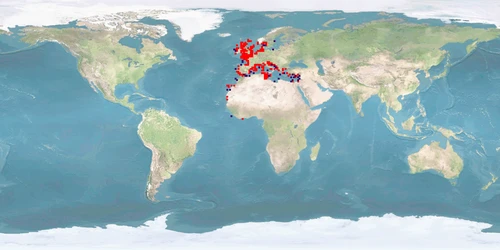
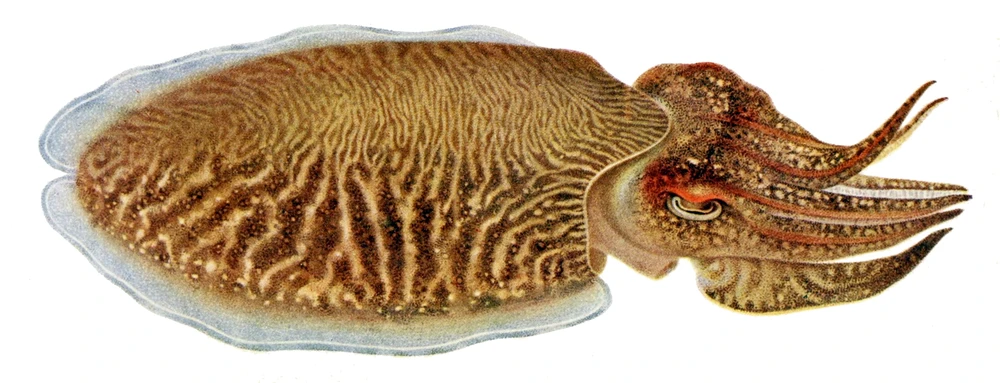
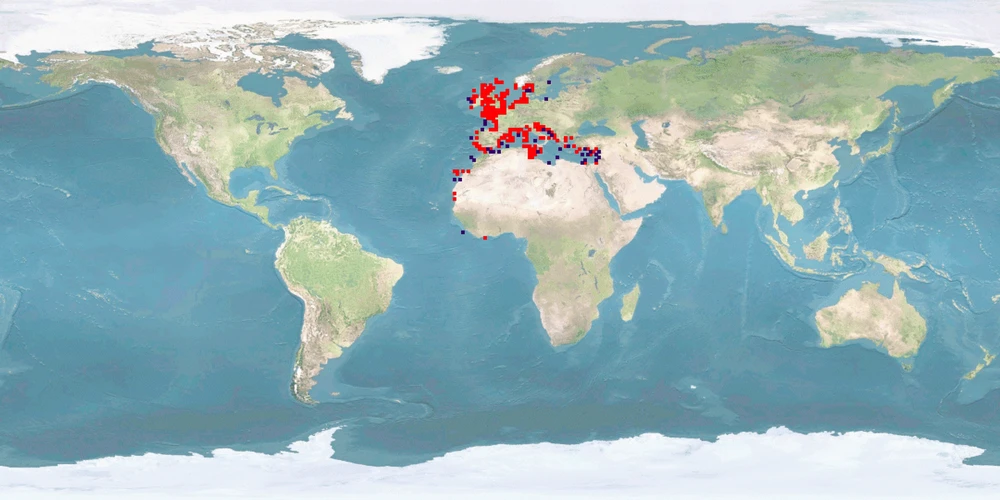
Sepia officinalis is mostly found in the eastern Atlantic and in the Mediterranean Sea, attaining interest for fisheries and high market value; and it is used as an animal model for biological and biomedical research, for aquaculture production, and also for public exhibition in aquariums. However, the main bottlenecks in the S. officinalis culture are reproduction, feeding, and nutrition, which need to be solved before applying them to an industrial scale for human consumption. Though it is already cultured for the complete life cycle in consecutively bred generations, wild IND (eggs) are still being introduced to improve genetic pools. Detrimental for welfare in captivity are the seasonal migrations (mainly vertical) S. officinalis undertakes in the wild and that the current farming techniques result in high stress due to high densities, aggregations, and food and mate competitions, which consequently increase aggression at different life stages. In addition, cuttlefish skin is particularly sensitive and can be easily damaged during handling, transportation or stressful conditions due to confinement, and a humane slaughtering protocol is not yet established.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a high amount of evidence.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a high amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of these circumstances?
It is low for minimal farming conditions. It is high for high-standard farming conditions. Our conclusion is based on a high amount of evidence.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions. It is high for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a high amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is unclear for minimal and high-standard farming conditions. Our conclusion is based on a low amount of evidence.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 4 49, level 5 being fully domesticated.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes: WILD: carnivorous; prey on crustaceans, FISHES, molluscs, polychaetes, and nemertean worms 6. FARM: live shrimps or frozen diets based on crustaceans or a mixture of crustaceans and fishes 7. Unsuccessful attempts of partly* replacing fish meal and fish oil with pelleted diets 51 52 53 54.
*partly = <51% – mostly = 51-99% – completely = 100%
Side note: Commercial relevance
How much is this species farmed annually?
0.04 t in 2022 55.
Glossary
BENTHIC = living at the bottom of a body of water, able to rest on the floor
DOMESTICATION LEVEL 4 = entire life cycle closed in captivity without wild inputs 50
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FISHES = using "fishes" instead of "fish" for more than one individual - whether of the same species or not - is inspired by Jonathan Balcombe who proposed this usage in his book "What a fish knows". By referring to a group as "fishes", we acknowledge the individuals with their personalities and needs instead of an anonymous mass of "fish".
IND = individuals
JUVENILES = fully developed but immature individuals
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening
NEKTONIC = horizontal movement by active swimming
PHOTOPERIOD = duration of daylight
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
WILD = setting in the wild
Bibliography
2 Guerra, Angel, Marcos Pérez-Losada, Francisco Rocha, and Andrés Sanjuan. 2001. Species differentiation of Sepia officinalis and Sepia hierredda (Cephalopoda: Sepiidae) based on morphological and allozyme analyses. Journal of the Marine Biological Association of the United Kingdom 81: 271–281. https://doi.org/10.1017/S0025315401003745.
3 Sykes, António V., Pedro M. Domingues, Maria Loyd, Anne Sommerfield, and José P. Andrade. 2003. The influence of culture density and enriched environments on the first stage culture of young cuttlefish, Sepia officinalis (Linnaeus, 1758). Aquaculture International 11: 531–544. https://doi.org/10.1023/B:AQUI.0000013262.15437.e4.
4 Abecasis, David, Pedro Afonso, Ron K. O’Dor, and Karim Erzini. 2013. Small MPAs do not protect cuttlefish (Sepia officinalis). Fisheries Research 147: 196–201. https://doi.org/10.1016/j.fishres.2013.05.004.
5 Forsythe, John, Phillip Lee, Leigh Walsh, and Tara Clark. 2002. The effects of crowding on growth of the European cuttlefish, Sepia officinalis Linnaeus, 1758 reared at two temperatures. Journal of Experimental Marine Biology and Ecology 269: 173–185. https://doi.org/10.1016/S0022-0981(02)00006-0.
6 Guerra, Ángel. 2006. Ecology of sepia offcinalis. Vie et Milieu 56: 97–107.
7 Sykes, António V., Pedro Domingues, and José Pedro Andrade. 2014. Sepia officinalis. In Cephalopod Culture, ed. José Iglesias, Lidia Fuentes, and Roger Villanueva, 175–204. Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-94-017-8648-5_11.
8 Fiorito, Graziano, Andrea Affuso, Jennifer Basil, Alison Cole, Paolo de Girolamo, Livia D’Angelo, Ludovic Dickel, et al. 2015. Guidelines for the Care and Welfare of Cephalopods in Research –A consensus based on an initiative by CephRes, FELASA and the Boyd Group. Laboratory Animals 49: 1–90. https://doi.org/10.1177/0023677215580006.
9 Forsythe, J. W., R. H. DeRusha, and R. T. Hanlon. 1994. Growth, reproduction and life span of Sepia officinalis (Cephalopoda: Mollusca) cultured through seven consecutive generations. Journal of Zoology 233: 175–192. https://doi.org/10.1111/j.1469-7998.1994.tb08582.x.
10 Roper, Clyde F. E., Michael J. Sweeney, and C. E. Nauen. 1984. FAO species catalogue. Vol. 3. Cephalopods of The World An Annotated and Illustrated Catalogue of Species of Interest to Fisheries. Vol. 3. FAO Fisheries Synopsis 125. Rome, Italy: Food and Agriculture Organization of the United Nations.
11 Koueta, N, and E Boucaud-Camou. 2003. Combined effects of photoperiod and feeding frequency on survival and growth of juvenile cuttlefish Sepia officinalis L. in experimental rearing. Journal of Experimental Marine Biology and Ecology 296: 215–226. https://doi.org/10.1016/S0022-0981(03)00322-8.
12 Sykes, A V, P M Domingues, M Correia, and J P Andrade. 2006. Cuttlefish culture - state of the art and future trends: 10.
13 Sykes, António V, Diana Pereira, Covadonga Rodríguez, António Lorenzo, and José P Andrade. 2013. Effects of increased tank bottom areas on cuttlefish (Sepia officinalis, L.) reproduction performance. Aquaculture Research 44: 1017–1028. https://doi.org/10.1111/j.1365-2109.2012.03106.x.
14 Gauvrit, E., R. Le Goff, and J. Daguzan. 1997. Reproductive cycle of the cuttlefish, Sepia officinalis (L.) in the nothern part of the Bay of Biscay. Journal of Molluscan Studies 63: 19–28. https://doi.org/10.1093/mollus/63.1.19.
15 Wang, Jianjun, Graham J Pierce, Peter R Boyle, Vincent Denis, Jean-Paul Robin, and Jose M Bellido. 2003. Spatial and temporal patterns of cuttlefish (Sepia officinalis) abundance and environmental influences – a case study using trawl fishery data in French Atlantic coastal, English Channel, and adjacent waters. ICES Journal of Marine Science 60: 1149–1158. https://doi.org/10.1016/S1054-3139(03)00118-8.
16 Batista, Marisa I., Célia M. Teixeira, and Henrique N. Cabral. 2009. Catches of target species and bycatches of an artisanal fishery: The case study of a trammel net fishery in the Portuguese coast. Fisheries Research 100: 167–177. https://doi.org/10.1016/j.fishres.2009.07.007.
17 Neves, A., H. Cabral, V. Sequeira, I. Figueiredo, T. Moura, and L.S. Gordo. 2009. Distribution patterns and reproduction of the cuttlefish, Sepia officinalis in the Sado estuary (Portugal). Journal of the Marine Biological Association of the United Kingdom 89: 579–584. https://doi.org/10.1017/S0025315409002677.
18 Ezzeddine-Najai, Soufia. 1997. Tagging of the cuttlefish, Sepia officinalis L. (Cephalopoda: Decapoda), in the Gulf of Tunis: 8.
19 Bloor, Isobel S.M., Victoria J. Wearmouth, Stephen P. Cotterell, Matthew J. McHugh, Nicolas E. Humphries, Emma L. Jackson, Martin J. Attrill, and David W. Sims. 2013. Movements and behaviour of European common cuttlefish Sepia officinalis in English Channel inshore waters: First results from acoustic telemetry. Journal of Experimental Marine Biology and Ecology 448: 19–27. https://doi.org/10.1016/j.jembe.2013.06.013.
20 Keller, Stefanie, Maria Valls, Manuel Hidalgo, and Antoni Quetglas. 2014. Influence of environmental parameters on the life-history and population dynamics of cuttlefish Sepia officinalis in the western Mediterranean. Estuarine, Coastal and Shelf Science 145: 31–40. https://doi.org/10.1016/j.ecss.2014.04.016.
21 Guerra, Angel, and Bernardino G Castro. 1988. On the life cycle of Sepia officinalis (Cephalopoda, Sepioidea) in the ria de Vigo (NW Spain): 11.
22 Laptikhovsky, Vladimir, Alp Salman, Bahadir Önsoy, and Tuncer Katagan. 2003. Fecundity of the common cuttlefish, Sepia officinalis L. (Cephalopoda, Sepiida): a new look at the old problem. Scientia Marina 67: 279–284. https://doi.org/10.3989/scimar.2003.67n3279.
23 Dunn, M. R. 1999. Aspects of the stock dynamics and exploitation of cuttlefish, Sepia officinalis (Linnaeus, 1758), in the English Channel. Fisheries Research 40: 277–293. https://doi.org/10.1016/S0165-7836(98)00223-9.
24 Sykes, A.V., E. Almansa, A. Lorenzo, and J.P. Andrade. 2009. Lipid characterization of both wild and cultured eggs of cuttlefish (Sepia officinalis L.) throughout the embryonic development. Aquaculture Nutrition 15: 38–53. https://doi.org/10.1111/j.1365-2095.2008.00566.x.
25 Sykes, António V, Daniel Quintana, and José P Andrade. 2014. The Effects of light intensity on growth and survival of cuttlefish (sepia officinalis) hatchlings and Juveniles. Aquaculture Research 45: 2032–2040. https://doi.org/10.1111/are.12150.
26 Domingues, Pedro M., António Sykes, and José P. Andrade. 2001. The use of Artemia sp. or mysids as food source for hatchlings of the cuttlefish (Sepia officinalis L.); effects on growth and survival throughout the life cycle. Aquaculture International 9: 319–331. https://doi.org/10.1023/A:1020416811568.
27 Domingues, Pedro M., António Sykes, and José P. Andrade. 2002. The effects of temperature in the life cycle of two consecutive generations of the cuttlefish Sepia officinalis (Linnaeus, 1758), cultured in the Algarve (South Portugal). Aquaculture International 10: 207–220. https://doi.org/10.1023/A:1022148802078.
28 Domingues, P., R. Poirier, L. Dickel, E. Almansa, A. Sykes, and J.P. Andrade. 2003. Effects of culture density and live prey on growth and survival of juvenile cuttlefish, Sepia officinalis. Aquaculture International 11: 225–242. https://doi.org/10.1023/A:1024803802486.
29 Correia, Miguel, Pedro M. Domingues, António Sykes, and José P. Andrade. 2005. Effects of culture density on growth and broodstock management of the cuttlefish, Sepia officinalis (Linnaeus, 1758). Aquaculture 245: 163–173. https://doi.org/10.1016/j.aquaculture.2004.12.017.
30 Hanlon, R T, Ament, and Gabr. 1999. Behavioral aspects of sperm competition in cuttlefish, Sepia officinalis (Sepioidea: Cephalopoda): 10.
31 Warnke, K. 1994. Some aspects of social interaction during feeding in Sepia officinals (Mollusca: Cephalopoda) hatched and reared in the laboratory: 7.
32 Domingues, P., and L. Márquez. 2010. Effects of culture density and bottom area on growth and survival of the cuttlefish Sepia officinalis (Linnaeus, 1758). Aquaculture International 18: 361–369. https://doi.org/10.1007/s10499-009-9249-3.
33 Boal, Jean Geary, Rebecca A Hylton, Susan A Gonzalez, and Roger T Hanlon. 1999. Effects of Crowding on the Social Behavior of Cuttlefish (Sepia officinalis) 38: 7.
34 Domingues, P. M., T. Kingston, A. Sykes, and J. P. Andrade. 2001. Growth of young cuttlefish, Sepia officinalis (Linnaeus 1758) at the upper end of the biological distribution temperature range. Aquaculture Research 32: 923–930. https://doi.org/10.1046/j.1365-2109.2001.00631.x.
35 Bloor, Isobel S.M., Martin J. Attrill, and Emma L. Jackson. 2013. A Review of the Factors Influencing Spawning, Early Life Stage Survival and Recruitment Variability in the Common Cuttlefish (Sepia officinalis). In Advances in Marine Biology, 65:1–65. Elsevier. https://doi.org/10.1016/B978-0-12-410498-3.00001-X.
36 Abecasis, D, P Afonso, and K Erzini. 2014. Combining multispecies home range and distribution models aids assessment of MPA effectiveness. Marine Ecology Progress Series 513: 155–169. https://doi.org/10.3354/meps10987.
37 Sykes, António V., Filipa D. Baptista, Rui A. Gonçalves, and José P. Andrade. 2012. Directive 2010/63/EU on animal welfare: a review on the existing scientific knowledge and implications in cephalopod aquaculture research: Cephalopod aquaculture research welfare. Reviews in Aquaculture 4: 142–162. https://doi.org/10.1111/j.1753-5131.2012.01070.x.
38 Hanley, J.S., Nadav Shashar, R. Smolowitz, W. Mebane, and Hanlon, R T. 1999. Soft-sided Tanks Improve Long-term Health of Cultured Cuttlefish 197: 237–238.
39 King, Alison J., and Shelley A. Adamo. 2006. The ventilatory, cardiac and behavioural responses of resting cuttlefish (Sepia officinalis L.) to sudden visual stimuli. Journal of Experimental Biology 209: 1101–1111. https://doi.org/10.1242/jeb.02116.
40 Sobrino, I, L Silva, J M Bellido, and F Ramos. 2002. Rainfall, river discharges and sea temperature as factors affecting abundance of two coastal benthic cephalopod species in the Gulf of Cádiz (SW Spain). BULLETIN OF MARINE SCIENCE 71: 15.
41 Sykes, António V, Pedro M Domingues, Lorenzo Márquez, and José P Andrade. 2011. The effects of tank colours on the growth and survival of cuttlefish (Sepia officinalis, Linnaeus 1758) hatchlings and juveniles: Optimizing cuttlefish growth and survival through tank colours. Aquaculture Research 42: 441–449. https://doi.org/10.1111/j.1365-2109.2010.02639.x.
42 Gonçalves, Rui A., Cláudia Aragão, Paulo A. Frias, and António V. Sykes. 2012. The use of different anaesthetics as welfare promoters during short-term human manipulation of European cuttlefish (Sepia officinalis) juveniles. Aquaculture 370–371: 130–135. https://doi.org/10.1016/j.aquaculture.2012.10.014.
43 Gutowska, Magdalena A., Frank Melzner, Hans O. Pörtner, and Sebastian Meier. 2010. Cuttlebone calcification increases during exposure to elevated seawater pCO2 in the cephalopod Sepia officinalis. Marine Biology 157: 1653–1663. https://doi.org/10.1007/s00227-010-1438-0.
44 Boyer, Christophe, Eric Maubert, Yves Charnay, and Raymond Chichery. 2007. Distribution of neurokinin A-like and serotonin immunoreactivities within the vertical lobe complex in Sepia officinalis. Brain Research 1133: 53–66. https://doi.org/10.1016/j.brainres.2006.11.042.
45 Wollesen, T., R. Loesel, and A. Wanninger. 2009. Pygmy squids and giant brains: Mapping the complex cephalopod CNS by phalloidin staining of vibratome sections and whole-mount preparations. Journal of Neuroscience Methods 179: 63–67. https://doi.org/10.1016/j.jneumeth.2009.01.021.
46 Bardou, Isabelle, Jérôme Leprince, Raymond Chichery, Hubert Vaudry, and Véronique Agin. 2010. Vasopressin/oxytocin-related peptides influence long-term memory of a passive avoidance task in the cuttlefish, Sepia officinalis. Neurobiology of Learning and Memory 93: 240–247. https://doi.org/10.1016/j.nlm.2009.10.004.
47 Talbot, Christopher M., and Justin N. Marshall. 2011. The retinal topography of three species of coleoid cephalopod: significance for perception of polarized light. Philosophical Transactions of the Royal Society B: Biological Sciences 366: 724–733. https://doi.org/10.1098/rstb.2010.0254.
48 Andrews, Paul L. R., Anne-Sophie Darmaillacq, Ngaire Dennison, Ian G. Gleadall, Penny Hawkins, John B. Messenger, Daniel Osorio, Valerie J. Smith, and Jane A. Smith. 2013. The identification and management of pain, suffering and distress in cephalopods, including anaesthesia, analgesia and humane killing. Journal of Experimental Marine Biology and Ecology 447. Cephalopod Biology a Special Issue Compiled under the Auspices of No-Profit Research Organization CephRes: 46–64. https://doi.org/10.1016/j.jembe.2013.02.010.
49 Arechavala-Lopez, Pablo. 2019. Personal communication.
50 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
51 Lee, Phillip G, John W Forsythe, F Paul DiMarco, Randal H DeRusha, and Roger T Hanlon. 1991. Initial palatability and growth trials on pelleted diets for cephalopods. Bulletin of Marine Science 49: 362–372.
52 Castro, Bernardino G, and Phillip G Lee. 1994. The effects of semi-purified diets on growth and condition of Sepia oficinalis L. (Mollusca: Cephalopoda). Comp. Biochem. Physiol. 109A: 1007–1016.
53 Domingues, Pedro, Ana Ferreira, Lorenzo Marquez, José P. Andrade, Nelda López, and Carlos Rosas. 2008. Growth, absorption and assimilation efficiency by mature cuttlefish (Sepia officinalis) fed with alternative and artificial diets. Aquaculture International 16: 215–229. https://doi.org/10.1007/s10499-007-9139-5.
54 Ferreira, A., L. Marquez, E. Almansa, J.P. Andrade, A. Lorenzo, and P.M. Domingues. 2009. The use of alternative diets to culture juvenile cuttlefish, Sepia officinalis: effects on growth and lipid composition: Alternative diets and lipid composition in S. officinalis. Aquaculture Nutrition 16: 262–275. https://doi.org/10.1111/j.1365-2095.2009.00661.x.
55 FAO. 2024. FishStat: Global aquaculture production 1950-2022. www.fao.org/fishery/en/statistics/software/fishstatj: FishStatJ.








Lorem ipsum
Something along the lines of: we were aware of the importance of some topics so that we wanted to include them and collect data but not score them. For WelfareChecks | farm, these topics are "domestication level", "feed replacement", and "commercial relevance". The domestication and commercial relevance aspects allow us to analyse the questions whether increasing rate of domestication or relevance in farming worldwide goes hand in hand with better welfare; the feed replacement rather goes in the direction of added suffering for all those species which end up as feed. For a carnivorous species, to gain 1 kg of meat, you do not just kill this one individual but you have to take into account the meat that it was fed during its life in the form of fish meal and fish oil. In other words, carnivorous species (and to a degree also omnivorous ones) have a larger "fish in:fish out" ratio.
Lorem ipsum
Probably, we updated the profile. Check the version number in the head of the page. For more information on the version, see the FAQ about this. Why do we update profiles? Not just do we want to include new research that has come out, but we are continuously developing the database itself. For example, we changed the structure of entries in criteria or we added explanations for scores in the WelfareCheck | farm. And we are always refining our scoring rules.
The centre of the Overview is an array of criteria covering basic features and behaviours of the species. Each of this information comes from our literature search on the species. If we researched a full Dossier on the species, probably all criteria in the Overview will be covered and thus filled. This was our way to go when we first set up the database.
Because Dossiers are time consuming to research, we switched to focusing on WelfareChecks. These are much shorter profiles covering just 10 criteria we deemed important when it comes to behaviour and welfare in aquaculture (and lately fisheries, too). Also, WelfareChecks contain the assessment of the welfare potential of a species which has become the main feature of the fair-fish database over time. Because WelfareChecks do not cover as many criteria as a Dossier, we don't have the information to fill all blanks in the Overview, as this information is "not investigated by us yet".
Our long-term goal is to go back to researching Dossiers for all species covered in the fair-fish database once we set up WelfareChecks for each of them. If you would like to support us financially with this, please get in touch at ffdb@fair-fish.net
See the question "What does "not investigated by us yet" mean?". In short, if we have not had a look in the literature - or in other words, if we have not investigated a criterion - we cannot know the data. If we have already checked the literature on a criterion and could not find anything, it is "no data found yet". You spotted a "no data found yet" where you know data exists? Get in touch with us at ffdb@fair-fish.net!
Once you have clicked on "show details", the entry for a criterion will unfold and display the summarised information we collected from the scientific literature – complete with the reference(s).
As reference style we chose "Springer Humanities (numeric, brackets)" which presents itself in the database as a number in a grey box. Mouse over the box to see the reference; click on it to jump to the bibliography at the bottom of the page. But what does "[x]-[y]" refer to?
This is the way we mark secondary citations. In this case, we read reference "y", but not reference "x", and cite "x" as mentioned in "y". We try to avoid citing secondary references as best as possible and instead read the original source ourselves. Sometimes we have to resort to citing secondarily, though, when the original source is: a) very old or not (digitally) available for other reasons, b) in a language no one in the team understands. Seldomly, it also happens that we are running out of time on a profile and cannot afford to read the original. As mentioned, though, we try to avoid it, as citing mistakes may always happen (and we don't want to copy the mistake) and as misunderstandings may occur by interpreting the secondarily cited information incorrectly.
If you spot a secondary reference and would like to send us the original work, please contact us at ffdb@fair-fish.net
In general, we aim at giving a good representation of the literature published on the respective species and read as much as we can. We do have a time budget on each profile, though. This is around 80-100 hours for a WelfareCheck and around 300 hours for a Dossier. It might thus be that we simply did not come around to reading the paper.
It is also possible, though, that we did have to make a decision between several papers on the same topic. If there are too many papers on one issue than we manage to read in time, we have to select a sample. On certain topics that currently attract a lot of attention, it might be beneficial to opt for the more recent papers; on other topics, especially in basic research on behaviour in the wild, the older papers might be the go-to source.
And speaking of time: the paper you are missing from the profile might have come out after the profile was published. For the publication date, please check the head of the profile at "cite this profile". We currently update profiles every 6-7 years.
If your paper slipped through the cracks and you would like us to consider it, please get in touch at ffdb@fair-fish.net
This number, for example "C | 2.1 (2022-11-02)", contains 4 parts:
- "C" marks the appearance – the design level – of the profile part. In WelfareChecks | farm, appearance "C" is our most recent one with consistent age class and label (WILD, FARM, LAB) structure across all criteria.
- "2." marks the number of major releases within this appearance. Here, it is major release 2. Major releases include e.g. changes of the WelfareScore. Even if we just add one paper – if it changes the score for one or several criteria, we will mark this as a major update for the profile. With a change to a new appearance, the major release will be re-set to 1.
- ".1" marks the number of minor updates within this appearance. Here, it is minor update 1. With minor updates, we mean changes in formatting, grammar, orthography. It can also mean adding new papers, but if these papers only confirm the score and don't change it, it will be "minor" in our book. With a change to a new appearance, the minor update will be re-set to 0.
- "(2022-11-02)" is the date of the last change – be it the initial release of the part, a minor, or a major update. The nature of the changes you may find out in the changelog next to the version number.
If an Advice, for example, has an initial release date and then just a minor update date due to link corrections, it means that – apart from correcting links – the Advice has not been updated in a major way since its initial release. Please take this into account when consulting any part of the database.
First up, you will find answers to questions for the specific page you are on. Scrolling down in the FAQ window, there are also answers to more general questions. Explore our website and the other sub pages and find there the answers to questions relevant for those pages.
In the fair-fish database, when you have chosen a species (either by searching in the search bar or in the species tree), the landing page is an Overview, introducing the most important information to know about the species that we have come across during our literatures search, including common names, images, distribution, habitat and growth characteristics, swimming aspects, reproduction, social behaviour but also handling details. To dive deeper, visit the Dossier where we collect all available ethological findings (and more) on the most important aspects during the life course, both biologically and concerning the habitat. In contrast to the Overview, we present the findings in more detail citing the scientific references.
Depending on whether the species is farmed or wild caught, you will be interested in different branches of the database.
Farm branch
Founded in 2013, the farm branch of the fair-fish database focuses on farmed aquatic species.
Catch branch
Founded in 2022, the catch branch of the fair-fish database focuses on wild-caught aquatic species.
The heart of the farm branch of the fair-fish database is the welfare assessment – or WelfareCheck | farm – resulting in the WelfareScore | farm for each species. The WelfareCheck | farm is a condensed assessment of the species' likelihood and potential for good welfare in aquaculture, based on welfare-related findings for 10 crucial criteria (home range, depth range, migration, reproduction, aggregation, aggression, substrate, stress, malformations, slaughter).
For those species with a Dossier, we conclude to-be-preferred farming conditions in the Advice | farm. They are not meant to be as detailed as a rearing manual but instead, challenge current farming standards and often take the form of what not to do.
In parallel to farm, the main element of the catch branch of the fair-fish database is the welfare assessment – or WelfareCheck | catch – with the WelfareScore | catch for each species caught with a specific catching method. The WelfareCheck | catch, too, is a condensed assessment of the species' likelihood and potential for good welfare – or better yet avoidance of decrease of good welfare – this time in fisheries. We base this on findings on welfare hazards in 10 steps along the catching process (prospection, setting, catching, emersion, release from gear, bycatch avoidance, sorting, discarding, storing, slaughter).
In contrast to the farm profiles, in the catch branch we assess the welfare separately for each method that the focus species is caught with. In the case of a species exclusively caught with one method, there will be one WelfareCheck, whereas in other species, there will be as many WelfareChecks as there are methods to catch the species with.
Summarising our findings of all WelfareChecks | catch for one species in Advice | catch, we conclude which catching method is the least welfare threatening for this species and which changes to the gear or the catching process will potentially result in improvements of welfare.
Welfare of aquatic species is at the heart of the fair-fish database. In our definition of welfare, we follow Broom (1986): “The welfare of an individual is its state as regards its attempts to cope with its environment.” Thus, welfare may be perceived as a continuum on which an individual rates “good” or “poor” or everything in between.
We pursue what could be called a combination of not only a) valuing the freedom from injuries and stress (function-based approach) but b) supporting attempts to provide rewarding experiences and cognitive challenges (feelings-based approach) as well as c) arguing for enclosures that mimic the wild habitat as best as possible and allow for natural behaviour (nature-based approach).
Try mousing over the element you are interested in - oftentimes you will find explanations this way. If not, there will be FAQ on many of the sub-pages with answers to questions that apply to the respective sub-page. If your question is not among those, contact us at ffdb@fair-fish.net.
It's right here! We decided to re-name it to fair-fish database for several reasons. The database has grown beyond dealing purely with ethology, more towards welfare in general – and so much more. Also, the partners fair-fish and FishEthoGroup decided to re-organise their partnership. While maintaining our friendship, we also desire for greater independence. So, the name "fair-fish database" establishes it as a fair-fish endeavour.