Information
Authors: Jenny Volstorf, Caroline Marques Maia, João L. Saraiva
Version: C | 2.1Published: 2024-12-31
- minor editorial changes plus new side note "Commercial relevance"
- profile update resulting in major editorial and content changes (changing the scoring in criteria 1-5, 7-10)
- transfer to consistent age class and label structure resulting in changed appearance
WelfareScore | farm
The score card gives our welfare assessments for aquatic species in 10 criteria.
For each criterion, we score the probability to experience good welfare under minimal farming conditions ("Likelihood") and under high-standard farming conditions ("Potential") representing the worst and best case scenario. The third dimension scores how certain we are of our assessments based on the number and quality of sources we found ("Certainty").
The WelfareScore sums just the "High" scores in each dimension. Although good welfare ("High") seems not possible in some criteria, there could be at least a potential improvement from low to medium welfare (indicated by ➚ and the number of criteria).
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
➚ = potential improvements not reaching "High" - Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10 per dimension)
General remarks
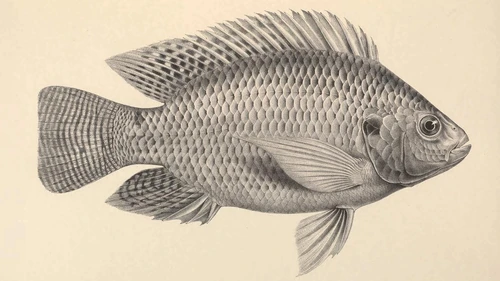
Oreochromis niloticus originated in fresh waters of the Middle East as well as sub-Saharan Africa. It is one of the top 5 most frequently cultured species worldwide, mainly reared in Southeast Asia, China, and Africa. Due to its fast growth, it can reach market size in 5-6 months. Its rearing does not come without a toll on the individuals, though. Because maturity sets in early as well, farmers in intensive rearing administer hormones through the feed that renders individuals all male, or genetic engineering results in all-male populations. This prevents unwanted reproduction and the growth deficiencies that come with it. Also, it favours males which grow up to 50% faster than females.
The two most frequently used culture systems are ponds and cages of which earthen ponds, especially irrigation ponds or reservoirs, overlap more with the natural needs of space, density, and substrate than cages. Shelters and shading may also be applied artifically, though. IND are allowed to spawn naturally, i.e., without manipulation and stripping, but there seems to be a trend towards hormonal induction to synchronise spawning or avoid injuries by aggressive SPAWNERS. Aggression is an issue in other age classes as well and does not seem to be avoidable completely; one recommended method – to size grade – is stressful itself. Other husbandry practices like handling and transport impose stress, too, which may be reduced but not avoided. Electrical stunning followed by exsanguination, evisceration, or filleting is availabla but at risk of failing if not executed correctly.
Note: we used some older sources that referred to O. niloticus as Tilapia nilotica 1 2 or Sarotherodon niloticus 3 4 5 6, as was common at the time.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
It is unclear for minimal and high-standard farming conditions, given we mainly found data from farms. Our conclusion is based on a medium amount of evidence, as further research is needed on home range in the wild.2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal farming conditions, as ponds, cages, tanks, and hapas do not cover the whole range in the wild (except for SPAWNERS). It is medium for high-standard farming conditions, as the mentioned systems at least overlap with the range in the wild. Our conclusion is based on a high amount of evidence unless farm studies show that O. niloticus is well under lower depths as in the wild.3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal farming conditions, as the species undertakes more or less extensive migrations, and we cannot be sure that providing each age class with their respective environmental conditions will satisfy their urge to migrate or whether they need to experience the transition. It is medium for high-standard farming conditions, as the space range in captivity overlaps with the migration distance. Our conclusion is based on a medium amount of evidence, as further research is needed on specific migration distances in the wild.4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of these circumstances?
It is low for minimal farming conditions, as the species is manipulated (hormonal manipulation, eggs taken from female’s mouth, in the lab also stripping) and may be taken from the wild. It is high for high-standard farming conditions, as natural breeding (without manipulation) with farm-reared IND is possible and verified for the farming context. Our conclusion is based on a medium amount of evidence, as further research is needed on reproduction behaviour in the wild.5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is low for minimal farming conditions, as densities in ponds, cages, tanks, and hapas go beyond the smallest density in the wild. It is medium for high-standard farming conditions, as densities in other ponds at least overlap with the density range in the wild. Our conclusion is based on a high amount of evidence, unless farm studies show that O. niloticus is well under higher densities as in the wild.6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal farming conditions, as the species is aggressive – even cannibalistic – in almost all age classes. It is medium for high-standard farming conditions, as a) ways to reduce (but not avoid) aggression (size homogeneity, density) are verified for the farming context, although there are contradictory findings on whether increasing or decreasing density is best and b) more ways to reduce aggression (adding oregano oil or tryptophan to the diet, tactile stimulation, low light intensity) need to be verified for the farming context. Our conclusion is based on a medium amount of evidence.7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions, as almost all age classes of the species use substrate, but some ponds, cages, tanks, and hapas are devoid of it. It is high for high-standard farming conditions given a) eggs are left in female’s mouth, b) earthen ponds for FRY to ADULTS which are not replaced by concrete bottom, and given b) natural reproduction with spawning substrate in ponds for SPAWNERS. Our conclusion is based on a medium amount of evidence, as further research is needed to determine whether shading and aerial protection nets suffice as replacements of structurally complex wetlands and macrophytes.8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions, as the species is stressed (water quality, handling, confinement, crowding, transport). It is medium for high-standard farming conditions, as some ways to reduce (but not avoid) stress are verified for the farming context. Our conclusion is based on a high amount of evidence, as it seems clear that stress cannot be avoided.9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is high for minimal and high-standard farming conditions, as malformation rates do not exceed 10%. Our conclusion is based on a medium amount of evidence, as further research is needed.10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is low for minimal farming conditions (asphyxia, hypothermia, live filleting). It is high for high-standard farming conditions, as electrical stunning, followed by exsanguination, evisceration, or filleting, induces unconsciousness fast (if done correctly), kills while still unconscious, and is verified for the farming context. Our conclusion is based on a medium amount of evidence, as further research is needed to determine how often electrical stunning fails in the farming context.Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 5 121, fully domesticated.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes:
- WILD: opportunistic – either mainly herbivorous 6 (non-native habitat: 45 44 49 122 88) or mainly omnivorous (non-native habitat: 8 48 43 50).
- FARM: fish meal may be completely* replaced by non-forage fishery components 123. Ponds: no external feed administered in extensive culture systems 11. Cages: feed without fish meal and fish oil 26. Higher growth of SPAWNERS in fertilised than unfertilised ponds, higher number of hatched FRY at 25-30% protein level for SPAWNERS in pre-spawning period 14.
- LAB: fish meal may be completely* replaced be some sustainable sources 124, not 125 126, partly* 127, or mostly* replaced by other sustainable sources 128. Fish meal may be partly* replaced by non-forage fishery components 129 130. Fish oil may be completely* replaced by some sustainable sources 131 132, partly* by other sustainable sources 133.
*partly = <51% – mostly = 51-99% – completely = 100%
Side note: Commercial relevance
How much is this species farmed annually?
4,590,292 t/year 1990-2019 amounting to estimated 8,657,000,000-13,568,000,000 IND/year 1990-2019 134.
Glossary
DOMESTICATION LEVEL 5 = selective breeding programmes are used focusing on specific goals 121
EURYHALINE = tolerant of a wide range of salinities
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FINGERLINGS = early juveniles with fully developed scales and working fins, the size of a human finger
FRY = larvae from external feeding on
IND = individuals
JUVENILES = fully developed but immature individuals
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening
MOUTHBROODER = also "mouthbreeder"; parent taking eggs into the mouth after fertilisation
NTU = Nephelometric Turbidity Units
PHOTOPERIOD = duration of daylight
POTAMODROMOUS = migrating within fresh water
RAS = Recirculating Aquaculture System - almost completely closed system using filters to clean and recirculate water with the aim of reducing water input and with the advantage of enabling close control of environmental parameters to maintain high water quality
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
TOTAL LENGTH = from snout to tip of caudal fin as compared to fork length (from snout to fork of caudal fin) 41 or standard length (from head to base of tail fin) or body length (from the base of the eye notch to the posterior end of the telson)
WILD = setting in the wild
Bibliography
2 Lowe-McConnell, Rosemary H. 1959. Breeding behaviour patterns and ecological differences between tilapia species and their significance for evolution within the genus tilapia (Pisces: Cichlidae). Proceedings of the Zoological Society of London 132: 1–30. https://doi.org/10.1111/j.1469-7998.1959.tb05510.x.
3 Hopson, A. J., and J. Hopson. 1982. The fishes of Lake Turkana. In A report on the findings of the Lake Turkana project 1972-1975, ed. A. J. Hopson, 1:281–348. London: Overseas Development Administration.
4 Hopson, A. J., B. J. Harbott, and A. A. Q. R McLeod. 1982. Shore survey. In A report on the findings of the Lake Turkana project 1972-1975, ed. A. J. Hopson, 2:557–580. London: Overseas Development Administration.
5 Hopson, A. J., J. Hopson, A. A. Q. R McLeod, and M. G. Pawson. 1982. Openwater surveys. In A report on the findings of the Lake Turkana project 1972-1975, ed. A. J. Hopson, 2:581–750. London: Overseas Development Administration.
6 Hopson, A. J., A. A. Q. R McLeod, B. J. Harbott, and J. T. N. Ogari. 1982. The biology of perciform fishes. In A report on the findings of the Lake Turkana project 1972-1975, ed. A. J. Hopson, 5:1283–1502. London: Overseas Development Administration.
7 Komolafe, O. O., and G. a. O. Arawomo. 2007. Reproductive strategy of Oreochromis niloticus (Pisces: Cichlidae) in Opa reservoir, Ile-Ife, Nigeria. Revista de Biología Tropical 55: 595–602.
8 Balirwa, J. S. 1998. Lake Victoria wetlands and the ecology of the Nile tilapia, Oreochromis niloticus Linne. Ph.D. dissertation, Wageningen, The Netherlands: Wageningen Agricultural University.
9 Peterson, Mark S., William T. Slack, Nancy J. Brown-Peterson, Jennifer L. McDonald, and C. M. Taylor. 2004. Reproduction in Nonnative Environments: Establishment of Nile Tilapia, Oreochromis niloticus, in Coastal Mississippi Watersheds. Copeia 2004: 842–849. https://doi.org/10.1643/CE-04-134R1.
10 Lowe (McConnell), Rosemary H. 1955. The Feduncity of Tilapia Species. The East African Agricultural Journal 21: 45–52.
11 Salin, Krishna R., and Amara Yakupitiyage. 2023. A manual for Nile tilapia seed production and grow-out aquaculture. Penang, Malaysia: WorldFish.
12 Soltan, M. A., A. Z. Hegazi, S. M. M. El-Laithy, and A. F. Fath El-Bab. 2007. Effect of spawning season, male and female body weight on reproductive performance of Nile tilapia, Oreochromis niloticus. Journal of the Egyptian Veterinary Medical Association 67: 97–108.
13 Fessehaye, Yonas, Zizy El-bialy, Mahmoud A. Rezk, Richard Crooijmans, Henk Bovenhuis, and Hans Komen. 2006. Mating systems and male reproductive success in Nile tilapia (Oreochromis niloticus) in breeding hapas: A microsatellite analysis. Aquaculture 256: 148–158. https://doi.org/10.1016/j.aquaculture.2006.02.024.
14 Fayed, W., A. El-Dahhar, S. El-Zaeem, Z. El-Greisy, M. Salama, and G. Sallam. 2014. Influence of Protein Levels and Pond Fertilization during Broodstock Pre-Spawning Period on the Tolerance of Nile Tilapia, Oreochromis niloticus, Fry to Winter Season Temperature. Mediterranean Aquaculture Journal 6: 1–13. https://doi.org/10.21608/maj.2014.4623.
15 El-Sayed Farag, Mohamed. 2003. Reporoductive performance of Oreochromis niloticus through three seasons in eaarthen ponds. Egyptian Journal of Aquatic Biology and Fisheries 7: 263–282. https://doi.org/10.21608/ejabf.2003.1794.
16 Maia, C.M. 2024. Personal communication.
17 Saboya, J. P. S., G. S. Araujo, J. W. A. Silva, J. Sousa Junior, R. L. Maciel, and W. R. L. Farias. 2012. Efeito dos polissacarídeos sulfatados da rodofícea Kappaphycus alvarezii em pós-larvas de tilápia do Nilo (Oreochromis niloticus) submetidas a situações de estresse. Acta Scientiarum. Animal Sciences 34: 215–221. https://doi.org/10.4025/actascianimsci.v34i3.13213.
18 El-Sayed, A-F M, A El-Ghobashy, and M Al-Amoudi. 1996. Effects of pond depth and water temperature on the growth, mortality and body composition of Nile tilapia, Oreochromis niloticus (L.). Aquaculture Research 27: 681–687. https://doi.org/10.1046/j.1365-2109.1996.00776.x.
19 Gilbert, P. 1996. Breeding and propagation of tilapia (Oreochromis niloticus) in a floating hatchery, Gabon. Naga, the ICLARM Quarterly 19: 26–33.
20 Abaho, Ivan, Thaddeus Zaabwe, Andrew Izaara, Howard N. Kasigwa, Norman Mushabe, Steven Byenkya, Mujibu Nkambo, Sylvester D. Baguma, David L. N. Hafashimana, and Jackson Efitre. 2020. Effect of stocking density on growth and survival of Nile tilapia (Oreochromis niloticus, Linnaeus 1758) under cage culture in Lake Albert, Uganda. International Journal of Fisheries and Aquaculture 12: 26–35. https://doi.org/10.5897/IJFA2018.0671.
21 Romana-Eguia, Maria Rowena R., Ruel V. Eguia, and Rolando V. Pakingking, jr. 2020. Tilapia culture: the basics. Tigbauan, Iloilo, Philippines: Training and Information Division, Southeast Asian Fisheries Development Center Aquaculture Department.
22 Abou, Youssouf, Emile D Fiogbé, and Jean-Claude Micha. 2007. Effects of stocking density on growth, yield and profitability of farming Nile tilapia, Oreochromis niloticus L., fed Azolla diet, in earthen ponds. Aquaculture Research 38: 595–604. https://doi.org/10.1111/j.1365-2109.2007.01700.x.
23 Agyakwah, Seth K., Ruby Asmah, Emmanual T. D. Mensah, Catherine Ragasa, Sena Amewu, Nhuong Tran, Mathew Oyih, and Peter Ziddah. 2020. Farmer’s manual on small-scale tilapia pond farming in Ghana.
24 Xu, Pao, and Junchao Ming. 2018. Status and Trends of the Tilapia Farming Industry Development. In Aquaculture in China: Success stories and modern trends, 404–420. John Wiley & Sons, Ltd.
25 Shoko, Amon Paul, Samwel Mchele Limbu, Hillary Deogratias John Mrosso, Adolf Faustine Mkenda, and Yunus Daud Mgaya. 2016. Effect of stocking density on growth, production and economic benefits of mixed sex Nile tilapia (Oreochromis niloticus) and African sharptooth catfish (Clarias gariepinus) in polyculture and monoculture. Aquaculture Research 47: 36–50. https://doi.org/10.1111/are.12463.
26 Campagnolo, R., A. Freccia, R. R. Bergmann, F. Meurer, and R. A. Bombardelli. 2013. Óleos essenciais na alimentação de alevinos de tilápia do Nilo. Revista Brasileira de Saúde e Produção Animal 14: 565–573.
27 Araujo, G. S., J. A. G. Rodrigues, J. W. A. Silva, and W. R. L. Farias. 2010. Cultivo da tilápia do Nilo em tanques-rede circulares em diferentes densidades de estocagem. Bioscience Journal 26: 428–434. Brazil; Contemporany.
28 Garcia, Fabiana, Daiane M. Romera, Kátia S. Gozi, Eduardo M. Onaka, Fernando S. Fonseca, Sérgio H. C. Schalch, Pedro G. Candeira, et al. 2013. Stocking density of Nile tilapia in cages placed in a hydroelectric reservoir. Aquaculture 410–411: 51–56. https://doi.org/10.1016/j.aquaculture.2013.06.010.
29 Khairnar, Sachin Onkar, A. H. Shanthanagouda, and S. Vijay Kumar Reddy. 2020. Influence of stocking density on growth and physiological responses of Nile tilapia (GIFT strain) in cages. J. Exp. Zool. India 23: 731–735.
30 Costa, Â. A. P., R. Roubach, B. S. L. Dallago, G. W. Bueno, C. McManus, and F. E. M. Bernal. 2017. Influence of stocking density on growth performance and welfare of juvenile tilapia (Oreochromis niloticus) in cages. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 69: 243–251. https://doi.org/10.1590/1678-4162-8939.
31 Siddiqui, A. Q., M. S. Howlader, and A. B. Adam. 1989. Culture of Nile tilapia, Oreochromis niloticus (L.), at three stocking densities in outdoor concrete tanks using drainage water. Aquaculture Research 20: 49–58. https://doi.org/10.1111/j.1365-2109.1989.tb00440.x.
32 Rakocy, J.E. 2005. Cultured Aquatic Species Information Programme. Oreochromis niloticus. Rome: FAO Fisheries and Aquaculture Department.
33 Wang, Kui, Kang Li, Liping Liu, Cristina Tanase, Rainier Mols, and Michiel van der Meer. 2023. Effects of light intensity and photoperiod on the growth and stress response of juvenile Nile tilapia (Oreochromis niloticus) in a recirculating aquaculture system. Aquaculture and Fisheries 8: 85–90. https://doi.org/10.1016/j.aaf.2020.03.001.
34 Brito, L. O., B. R. Simão, J. B. Pereira Neto, G. Cemirames, and C. M. S. B. de Azevedo. 2017. Densidade plantonica do policultivo de Litopenaeus vannamei. Ciência Animal Brasileira 18: e-16840.
35 McDonald, Jennifer L., Mark S. Peterson, and William T. Slack. 2007. Morphology, Density, and Spatial Patterning of Reproductive Bowers in an Established Alien Population of Nile Tilapia, Oreochromis niloticus. Journal of Freshwater Ecology 22: 461–468. https://doi.org/10.1080/02705060.2007.9664176.
36 Bandara, K.V. Sandun N., and Upali S. Amarasinghe. 2017. Factors Related to Nesting Sites of Oreochromis niloticus (Linnaeus 1758; Cichlidae) in Irrigation Reservoirs, Sri Lanka. Asian Fisheries Science 30: 319–335. https://doi.org/10.33997/j.afs.2017.30.4.008.
37 Turner, G. F., and R. L. Robinson. 2000. Reproductive biology, mating systems and parental care. In Tilapias: Biology and Exploitation, ed. Malcolm C. M. Beveridge and Brendan J. McAndrew, 33–58. Dordrecht: Springer Netherlands.
38 El-Ebiary, El Sayed H., Nabil M. Eweedah, El Sayed M. Abdel-Raouf, Ragab M. Abdel-Regal, and Basem S. Abdel-Aty. 2013. Comparative Study between the Performances of Nile Tilapia Oreochromisniloticus during and Out of the Normal Spawning Season. ANGLISTICUM. Journal of the Association-Institute for English Language and American Studies 3.
39 Bautista, A. M., M. H. Carlos, and A. I. San Antonio. 1988. Hatchery production of Oreochromis niloticus L. at different sex ratios and stocking densities. Aquaculture 73: 85–89. https://doi.org/10.1016/0044-8486(88)90043-9.
40 Vera Cruz, Emmanuel M., Eddie Boy T. Jimenez, and Zaldy P. Bartolome. 2023. Shading Influenced Water Quality and Seed Production of Nile Tilapia (Oreochromis niloticus L.) in the Hapa-within-Pond System During Warm Months. The Philippine Journal of Fisheries 30: 155–161. https://doi.org/10.31398/tpjf/30.2.2022-0026.
41 Pawson, M.G., and G.D. Pickett. 1996. The Annual Pattern of Condition and Maturity in Bass, Dicentrarchus Labrax, in Waters Around England and Wales. Journal of the Marine Biological Association of the United Kingdom 76: 107. https://doi.org/10.1017/S0025315400029040.
42 Balirwa, J. S. 1990. The present status of the fishery for tilapia in Lake Victoria (Uganda). In , 61–64.
43 Njiru, M., J. E. Ojuok, J. B. Okeyo-Owuor, M. Muchiri, M. J. Ntiba, and I. G. Cowx. 2006. Some biological aspects and life history strategies of Nile tilapia Oreochromis niloticus (L.) in Lake Victoria, Kenya: Biology of Nile tilapia in Lake Victoria. African Journal of Ecology 44: 30–37. https://doi.org/10.1111/j.1365-2028.2006.00610.x.
44 Goudswaard, K., J.H. Wanink, F. Witte, E.F.B. Katunzi, M.R. Berger, and D.J. Postma. 2004. Diel vertical migration of major fish-species in Lake Victoria, East Africa. Hydrobiologia 513: 141–152. https://doi.org/10.1023/B:hydr.0000018179.80116.93.
45 Bwanika, G. N., B. Makanga, Y. Kizito, L. J. Chapman, and J. Balirwa. 2004. Observations on the biology of Nile tilapia, Oreochromis niloticus L., in two Ugandan crater lakes. African Journal of Ecology 42: 93–101. https://doi.org/10.1111/j.1365-2028.2004.00468.x.
46 van Oijen, M. J. P. 1995. Appendix I. Key to Lake Victoria fishes other than haplochromine cichlids. In Fish Stocks and Fisheries of Lake Victoria: A Handbook for Field Observations, ed. Frans Witte and Wim L. T. van Densen, 209–300. Dyfed, Great Britain: Samara Publishing Limited.
47 Froese, R., and D. Pauly. 2014. FishBase. World Wide Web electronic publication. www.fishbase.org.
48 Bwanika, G. N., L. J. Chapman, Y. Kizito, and J. Balirwa. 2006. Cascading effects of introduced Nile perch (Lates niloticus) on the foraging ecology of Nile tilapia (Oreochromis niloticus). Ecology of Freshwater Fish 15: 470–481. https://doi.org/10.1111/j.1600-0633.2006.00185.x.
49 Oso, J. A., I. A. Ayodele, and O. Fagbuaro. 2006. Food and Feeding Habits of Oreochromis niloticus (L.) and Sarotherodon galilaeus (L.) in a Tropical Reservoir. World Journal of Zoology 1: 118–121.
50 Peterson, Mark S., William T. Slack, Gretchen L. Waggy, Jeremy Finley, Christa M. Woodley, and Melissa L. Partyka. 2006. Foraging in Non-Native Environments: Comparison of Nile Tilapia and Three Co-Occurring Native Centrarchids in Invaded Coastal Mississippi Watersheds. Environmental Biology of Fishes 76: 283–301. https://doi.org/10.1007/s10641-006-9033-4.
51 Uddin, M. S., A. Farzana, M. K. Fatema, M. E. Azim, M. A. Wahab, and M. C. J. Verdegem. 2007. Technical evaluation of tilapia (Oreochromis niloticus) monoculture and tilapia–prawn (Macrobrachium rosenbergii) polyculture in earthen ponds with or without substrates for periphyton development. Aquaculture 269: 232–240. https://doi.org/10.1016/j.aquaculture.2007.05.038.
52 Melo, Luis Henrique, Yuri Simões Martins, Rafael Magno Costa Melo, Paula Suzanna Prado, Ronald Kennedy Luz, Nilo Bazzoli, and Elizete Rizzo. 2019. Low salinity negatively affects early larval development of Nile tilapia, Oreochromis niloticus: insights from skeletal muscle and molecular biomarkers. Zygote 27: 375–381. https://doi.org/10.1017/S0967199419000431.
53 Lung’ayia, H. B. O. 1994. Some aspects of the reproductive biology of the Nile tilapia Oreochromis niloticus (L) in the Nyanza Gulf of Lake Victoria, Kenya. In Proceedings of the Second EEC Regional Seminar on Recent Trends of Research on Lake Victoria Fisheries, ed. E. Okemwa, E. O. Wakwabi, and A. Getabu, 121–127. Nairobi: ICIPE SCIENCE.
54 Souza, U. N., V. O. Felizardo, R. T. F. Freitas, C. C. V. Melo, M. R. Ferreira, and R. V. Reis-Neto. 2016. Influência do horário de aplicação e da variedade genética em fêmeas de tilápias Oreochromis niloticus submetidas à indução hormonal com hCG. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 68: 215–223. https://doi.org/10.1590/1678-4162-7716.
55 Heinrich, Wolfgang. 1967. Untersuchungen zum Sexualverhalten in der Gattung Tilapia (Cichlidae, Teleostei) und bei Artbastarden. Advances in Ethology 6: 684–754.
56 Uchida, Hiroshi, Satoshi Ogawa, Mina Harada, Masato Matushita, Munehico Iwata, Yasuo Sakuma, and Ishwar S. Parhar. 2005. The olfactory organ modulates gonadotropin-releasing hormone types and nest-building behavior in the tilapia Oreochromis niloticus. Journal of Neurobiology 65: 1–11. https://doi.org/10.1002/neu.20156.
57 Castro, A. L. S., E. Gonçalves-de-Freitas, G. L. Volpato, and C. Oliveira. 2009. Visual communication stimulates reproduction in Nile tilapia, Oreochromis niloticus (L.). Brazilian Journal of Medical and Biological Research 42: 368–374. https://doi.org/10.1590/S0100-879X2009000400009.
58 Rana, Kausik J. 1986. Parental influences on egg quality, fry production and fry performance in Oreochromis niloticus (Linnaeus) and O. mossambicus (Peters). Doctoral dissertation, U.K.: University of Stirling.
59 Volpato, G. L., C. R. A. Duarte, and A. C. Luchiari. 2004. Environmental color affects Nile tilapia reproduction. Brazilian Journal of Medical and Biological Research 37: 479–483. https://doi.org/10.1590/S0100-879X2004000400004.
60 Fernandes, AFA, ÉR Alvarenga, DAA Oliveira, CG Aleixo, Sa Prado, RK Luz, NLAF Sarmento, EA Teixeira, MR Luz, and EM Turra. 2013. Production of Oocytes of Nile Tilapia (Oreochromis niloticus) for In Vitro Fertilization via Hormonal Treatments. Reproduction in Domestic Animals 48: 1049–1055. https://doi.org/10.1111/rda.12212.
61 Shokr, El-Sayed A. M. S. 2015. Effect of Follicular Stimulating Hormone and Leutinizing Hormone on Reproduction, Physiological and Biochemical Changes of Oreochromis niloticus. Egyptian Academic Journal of Biological Sciences. C, Physiology and Molecular Biology 7: 61–73. https://doi.org/10.21608/eajbsc.2015.13703.
62 Piamsomboon, Patharapol, Nicole Sirisopit Mehl, Sudson Sirivaidyapong, and Janenuj Wongtavatchai. 2019. Assisted reproduction in Nile tilapia Oreochromis niloticus: Milt preservation, spawning induction and artificial fertilization. Aquaculture 507: 139–143. https://doi.org/10.1016/j.aquaculture.2019.04.019.
63 Rana, Krishen. 1988. Reproductive Biology and the Hatchery Rearing of Tilapia Eggs and Fry. In Recent Advances in Aquaculture, ed. James F. Muir and Ronald J. Roberts, 343–406. Dordrecht: Springer Netherlands.
64 Vera Cruz, E. M., and G. C. Mair. 1994. Conditions for effective androgen sex reversal in Oreochromis niloticus (L.). Aquaculture 122: 237–248. https://doi.org/10.1016/0044-8486(94)90513-4.
65 Lima, E. C. R., R. L. Souza, X. F. Wambach, U. L. Silva, and E. S. Correia. 2015. Cultivo da tilápia do Nilo Oreochromis niloticus em sistema de bioflocos com diferentes densidades de estocagem. Revista Brasileira de Saúde e Produção Animal 16: 948–957. https://doi.org/10.1590/S1519-99402015000400018.
66 Carvalho, E. D., A. L. S. Camargo, and A. S. Zanatta. 2010. Desempenho produtivo da tilápia do nilo em tanques-rede numa represa pública: modelo empírico de classificação. Ciência Rural 40: 1616–1622. https://doi.org/10.1590/S0103-84782010000700021.
67 Zhao, Jian, Zhaobin Gu, Mingming Shi, Huanda Lu, Jianping Li, Mingwei Shen, Zhangying Ye, and Songming Zhu. 2016. Spatial behavioral characteristics and statistics-based kinetic energy modeling in special behaviors detection of a shoal of fish in a recirculating aquaculture system. Computers and Electronics in Agriculture 127: 271–280. https://doi.org/10.1016/j.compag.2016.06.025.
68 Faller, U., and L. Debacker. 1988. Density-dependent behavioral shift in Oreochromis niloticus. In ICLARM Conference Proceedings (Philippines). ICLARM.
69 Barcellos, Leonardo José Gil, S. Nicolaiewsky, S M G. De Souza, and F. Lulhier. 1999. The effects of stocking density and social interaction on acute stress response in Nile tilapia Oreochromis niloticus (L.) fingerlings. Aquaculture Research 30: 887–892. https://doi.org/10.1046/j.1365-2109.1999.00419.x.
70 Delcourt, Johann, Christophe Becco, Nicolas Vandewalle, and Pascal Poncin. 2009. A video multitracking system for quantification of individual behavior in a large fish shoal: Advantages and limits. Behavior Research Methods 41: 228–235. https://doi.org/10.3758/BRM.41.1.228.
71 El-Hawarry, Waleed N., Radi A. Mohamed, and Safinaz A. Ibrahim. 2018. Collaborating effects of rearing density and oregano oil supplementation on growth, behavioral and stress response of Nile tilapia (Oreochromis niloticus). Egyptian Journal of Aquatic Research 44: 173–178. https://doi.org/10.1016/j.ejar.2018.06.008.
72 Gauy, Ana Carolina dos Santos, Marcela Cesar Bolognesi, and Eliane Gonçalves-de-Freitas. 2023. Body Tactile Stimulation Reduces the Effects of High Stocking Density on the Welfare of Nile Tilapia (Oreochromis niloticus). Fishes 8: 320. https://doi.org/10.3390/fishes8060320.
73 Saraiva, João L. 2017. Personal communication.
74 Barbosa, J. M., S. S. S. Brugiolo, J. Carolsfeld, and S. S. Leitão. 2006. Heterogeneous growth fingerlings of the Nile tilapia Oreochromis niloticus: effects of density and initial size variability. Brazilian Journal of Biology 66: 537–541. https://doi.org/10.1590/S1519-69842006000300020.
75 Evans, Joyce J., David J. Pasnik, Patrick Horley, Kimberly Kraeer, and Phillip H. Klesius. 2008. Aggression and Mortality among Nile Tilapia (Oreochromis niloticus) Maintained in the Laboratory at Different Densities. Research Journal of Animal Sciences 2: 57–64.
76 Barreto, Rodrigo Egydio, Graziele G. Arantes Carvalho, and Gilson Luiz Volpato. 2011. The aggressive behavior of Nile tilapia introduced into novel environments with variation in enrichment. Zoology 114: 53–57. https://doi.org/10.1016/j.zool.2010.09.001.
77 Pinho-Neto, Candido Ferreira, Caio Akira Miyai, Fabio Henrique Carretero Sanches, Percilia Cardoso Giaquinto, Helton Carlos Delicio, Leonardo José Gil Barcellos, Gilson Luiz Volpato, and Rodrigo Egydio Barreto. 2014. Does sex influence intraspecific aggression and dominance in Nile tilapia juveniles? Behavioural Processes 105: 15–18. https://doi.org/10.1016/j.beproc.2014.02.003.
78 Vieira, Bruna Renata Meletti, Isabela Inforzato Guermandi, Marina Sanson Bellot, Bruno Camargo-dos-Santos, João Favero-Neto, and Percília Cardoso Giaquinto. 2021. The effects of tryptophan supplementation on stress and aggression in Nile tilapia. Journal of Applied Ichthyology 37: 578–584. https://doi.org/10.1111/jai.14186.
79 Neto, João Favero, and Percilia Cardoso Giaquinto. 2020. Environmental enrichment techniques and tryptophan supplementation used to improve the quality of life and animal welfare of Nile tilapia. Aquaculture Reports 17: 100354. https://doi.org/10.1016/j.aqrep.2020.100354.
80 Gauy, Ana Carolina dos Santos, Marcela Cesar Bolognesi, and Eliane Gonçalves-de-Freitas. 2022. Long-term body tactile stimulation reduces aggression and improves productive performance in Nile tilapia groups. Scientific Reports 12: 20239. https://doi.org/10.1038/s41598-022-24696-3.
81 Volpato, G. L., A. C. Luchiari, C. R. A. Duarte, R. E. Barreto, and G. C. Ramanzini. 2003. Eye color as an indicator of social rank in the fish Nile tilapia. Brazilian Journal of Medical and Biological Research 36: 1659–1663. https://doi.org/10.1590/S0100-879X2003001200007.
82 Corrêa, S. A., M. O. Fernandes, K. K. Iseki, and J. A. Negrão. 2003. Effect of the establishment of dominance relationships on cortisol and other metabolic parameters in Nile tilapia (Oreochromis niloticus). Brazilian Journal of Medical and Biological Research 36: 1725–1731. https://doi.org/10.1590/S0100-879X2003001200015.
83 Barreto, Rodrigo Egydio, and Gilson Luiz Volpato. 2006. Ventilatory frequency of Nile tilapia subjected to different stressors. Journal of Experimental Animal Science 43: 189–196. https://doi.org/10.1016/j.jeas.2006.05.001.
84 Gonçalves-de-Freitas, Eliane, and Aline Chimello Ferreira. 2004. Female social dominance does not establish mating priority in Nile tilapia. Revista de Etologia 6: 33–37.
85 Carvalho, Thaís B., Francine Z. Mendonça, Roselene S. Costa-Ferreira, and Eliane Gonçalves-de-Freitas. 2013. The effect of increased light intensity on the aggressive behavior of the Nile tilapia, Oreochromis niloticus (Teleostei: Cichlidae). Zoologia (Curitiba) 30: 125–129. https://doi.org/10.1590/S1984-46702013000200001.
86 Mendonça, Francine Z., and Eliane Gonçalves-de-Freitas. 2008. Nest deprivation and mating success in Nile tilapia (Teleostei: Cichlidae). Revista Brasileira de Zoologia 25: 413–418. https://doi.org/10.1590/S0101-81752008000300005.
87 Longrie, Nicolas, Sam Van Wassenbergh, Pierre Vandewalle, Quentin Mauguit, and Eric Parmentier. 2009. Potential mechanism of sound production in Oreochromis niloticus (Cichlidae). Journal of Experimental Biology 212: 3395–3402. https://doi.org/10.1242/jeb.032946.
88 Zengeya, Tsungai Alfred, Anthony J. Booth, Armanda D. S. Bastos, and Christian T. Chimimba. 2011. Trophic interrelationships between the exotic Nile tilapia, Oreochromis niloticus and indigenous tilapiine cichlids in a subtropical African river system (Limpopo River, South Africa). Environmental Biology of Fishes 92: 479–489. https://doi.org/10.1007/s10641-011-9865-4.
89 Chapman, Lauren J., Colin A. Chapman, and Mark Chandler. 1996. Wetland ecotones as refugia for endangered fishes. Biological Conservation 78: 263–270. https://doi.org/10.1016/S0006-3207(96)00030-4.
90 Chapman, Lauren J., Colin A. Chapman, Richard Ogutu-Ohwayo, Mark Chandler, Les Kaufman, and Amanda E. Keiter. 1996. Refugia for Endangered Fishes from an Introduced Predator in Lake Nabugabo, Uganda. Conservation Biology 10: 554–561. https://doi.org/10.1046/j.1523-1739.1996.10020554.x.
91 Rosenberger, A. E., and L. J. Chapman. 1999. Hypoxic wetland tributaries as faunal refugia from an introduced predator. Ecology of Freshwater Fish 8: 22–34. https://doi.org/10.1111/j.1600-0633.1999.tb00049.x.
92 Schofield, Pamela J., and Lauren J. Chapman. 1999. Interactions Between Nile Perch, Lates niloticus, and Other Fishes in Lake Nabugabo, Uganda. Environmental Biology of Fishes 55: 343–358. https://doi.org/10.1023/A:1007544017989.
93 Tatemoto, P., G. Valença-Silva, Mariana R. Queiroz, and Donald M. Broom. 2021. Living with low environmental complexity increases fear indicators in Nile tilapia. Animal Behaviour 174: 169–174. https://doi.org/10.1016/j.anbehav.2021.02.006.
94 Saraiva, J. L., Margarida Nogueirinha, Rita Teodósio, Cláudia Aragão, Sofia Engrola, and Pablo Arechavala-Lopez. 2021. The effect of tank cover on welfare of farmed Nile tilapia. Applied Animal Behaviour Science: 105396. https://doi.org/10.1016/j.applanim.2021.105396.
95 Delicio, Helton Carlos, Rodrigo Egydio Barreto, Edvaldo Bento Normandes, Ana Carolina Luchiari, and Ana Lúcia Marcondes. 2006. A place preference test in the fish Nile tilapia. Journal of Experimental Animal Science 43: 141–148. https://doi.org/10.1016/j.jeas.2006.01.001.
96 Duponchelle, Fabrice, and Marc Legendre. 2001. Rapid phenotypic changes of reproductive traits in response to experimental modifications of spatial structure in Nile tilapia, Oreochromis niloticus. Aquatic Living Resources 14: 145–152. https://doi.org/10.1016/S0990-7440(01)01109-3.
97 Mendonça, F. Z., G. L. Volpato, R. S. Costa-Ferreira, and E. Gonçalves-de-Freitas. 2010. Substratum choice for nesting in male Nile tilapia Oreochromis niloticus. Journal of Fish Biology 77: 1439–1445. https://doi.org/10.1111/j.1095-8649.2010.02754.x.
98 Obirikorang, Kwasi Adu, Esther Adu Yeboah, Benjamin Apraku Gyampoh, and Seyram Kwadzo Amanie-Adjei. 2022. Effect of road conditions on physiological stress responses and post-transportation growth and survival of Nile tilapia (Oreochromis niloticus) fingerlings. Journal of Applied Aquaculture 34: 180–196. https://doi.org/10.1080/10454438.2020.1825269.
99 Moreira, A. G. L., A. A. C. Coelho, L. F. G. Albuquerque, R. T. Moreira, and W. R. L. Farias. 2015. Efeito do eugenol como agente mitigador do estresse no transporte de juvenis de tilápia do Nilo. Pesquisa Veterinária Brasileira 35: 893–898. https://doi.org/10.1590/S0100-736X2015001100004.
100 Barcellos, Leonardo José Gil, Gilson Luiz Volpato, Rodrigo Egydio Barreto, Ivanir Coldebella, and Daiane Ferreira. 2011. Chemical communication of handling stress in fish. Physiology & Behavior 103: 372–375. https://doi.org/10.1016/j.physbeh.2011.03.009.
101 Deriggi, Graziele Fernanda, Luis Antonio Kioshi Aoki Inoue, and Moraes Gilberto. 2006. Stress responses to handling in Nile tilapia (Oreochromis niloticus Linnaeus): assessment of eugenol as an alternative anesthetic = Respostas metabólicas da tilápia do Nilo (Oreochromis niloticus) submetida ao manuseio e ao anestésico eugenol. ResearchGate 28.
102 Simões, L. N., A. T. M. Gomide, V. M. F. Almeida-Val, A. L. Val, and L. C. Gomes. 2012. O uso do óleo de cravo como anestésico em juvenis avançados de tilápia do Nilo (Oreochromis niloticus). Acta Scientiarum. Animal Sciences 34: 175–181. https://doi.org/10.4025/actascianimsci.v34i2.13022.
103 Mustapha, Moshood Keke, Olatoyosi Taofeek Oladokun, Mubarak Mayowa Salman, Idris Adewale Adeniyi, and Dele Ojo. 2014. Does light duration (photoperiod) have an effect on the mortality and welfare of cultured Oreochromis niloticus and Clarias gariepinus? Turkish Journal of Zoology 38: 466–470.
104 Maia, Caroline Marques, and Gilson Luiz Volpato. 2013. Environmental light color affects the stress response of Nile tilapia. Zoology 116: 64–66. https://doi.org/10.1016/j.zool.2012.08.001.
105 Martins, Catarina I. M., Patricia I. M. Silva, Luis E. C. Conceição, Benjamin Costas, Erik Höglund, Øyvind Øverli, and Johan W. Schrama. 2011. Linking fearfulness and coping styles in fish. PLOS ONE 6: e28084. https://doi.org/10.1371/journal.pone.0028084.
106 Martins, Maurício Laterça, Daniela Takahashi Nomura, Danilo Makoto Yamaguchi Myiazaki, Fabiana Pilarsky, Karina Ribeiro, Marcello Pardi De Castro, and Cristiane Fátima Meldau De Campos. 2004. Physiological and haematological response of Oreochromis niloticus (Osteichthyes: Cichlidae) exposed to single and consecutive stress of capture. Acta Scientiarum. Animal Sciences 26: 449–456. https://doi.org/10.4025/actascianimsci.v26i4.1719.
107 Barreto, Rodrigo Egydio, and Gilson Luiz Volpato. 2007. Evaluating feeding as unconditioned stimulus for conditioning of an endocrine effect in Nile tilapia. Physiology & Behavior 92: 867–872. https://doi.org/10.1016/j.physbeh.2007.06.013.
108 Luchiari, A. C., and F. a. M. Freire. 2009. Effects of environmental colour on growth of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758), maintained individually or in groups. Journal of Applied Ichthyology 25: 162–167. https://doi.org/10.1111/j.1439-0426.2008.01203.x.
109 Barreto, Rodrigo Egydio, and Gilson Luiz Volpato. 2004. Caution for using ventilatory frequency as an indicator of stress in fish. Behavioural Processes 66: 43–51. https://doi.org/10.1016/j.beproc.2004.01.001.
110 Volpato, G. L., and R. E. Barreto. 2001. Environmental blue light prevents stress in the fish Nile tilapia. Brazilian Journal of Medical and Biological Research 34: 1041–1045. https://doi.org/10.1590/S0100-879X2001000800011.
111 Smith, Michael E., Andrew S. Kane, and Arthur N. Popper. 2004. Acoustical stress and hearing sensitivity in fishes: does the linear threshold shift hypothesis hold water? Journal of Experimental Biology 207: 3591–3602. https://doi.org/10.1242/jeb.01188.
112 Roques, Jonathan A. C., Wout Abbink, Femke Geurds, Hans van de Vis, and Gert Flik. 2010. Tailfin clipping, a painful procedure: Studies on Nile tilapia and common carp. Physiology & Behavior 101: 533–540. https://doi.org/10.1016/j.physbeh.2010.08.001.
113 Barreto, Rodrigo Egydio, and Gilson Luiz Volpato. 2006. Stress responses of the fish Nile tilapia subjected to electroshock and social stressors. Brazilian Journal of Medical and Biological Research 39: 1605–1612. https://doi.org/10.1590/S0100-879X2006001200012.
114 Fessehaye, Yonas, Hans Komen, Mahmoud A. Rezk, Johan A.M. van Arendonk, and Henk Bovenhuis. 2007. Effects of inbreeding on survival, body weight and fluctuating asymmetry (FA) in Nile tilapia, Oreochromis niloticus. Aquaculture 264: 27–35. https://doi.org/10.1016/j.aquaculture.2006.12.038.
115 Eissa, A.E., M. Moustafa, I.N. El-Husseiny, S. Saeid, O. Saleh, and T. Borhan. 2009. Identification of some skeletal deformities in freshwater teleosts raised in Egyptian aquaculture. Chemosphere 77: 419–425. https://doi.org/10.1016/j.chemosphere.2009.06.050.
116 Sonu. 2022. Developing high-welfare stunning solutions with Ace Aquatech. Regal Springs.
117 Volstorf, Jenny. 2024. Conclusion.
118 Lambooij, E., M. A. Gerritzen, H. Reimert, D. Burggraaf, and J. W. van de Vis. 2008. A humane protocol for electro-stunning and killing of Nile tilapia in fresh water. Aquaculture 275: 88–95. https://doi.org/10.1016/j.aquaculture.2008.01.026.
119 Oliveira Filho, Paulo Roberto Campagnoli de, Pamela Jenny Montes Girao, Mariza Pires de Melo, and Elisabete Maria Macedo Viegas. 2015. Indicators of stress in tilapia subjected to different stunning methods. Boletim do Instituto de Pesca, São Paulo 41: 335–343.
120 Gonzalez, Daniel Santiago Rucinque. 2021. Unconsciousness assessment through electroencephalography (EEG), in the process of stunning for humane slaughter of Nile tilapia. SÃO PAULO, Brazil: UNIVERSIDADE DE SÃO PAULO FACULDADE DE ZOOTECNIA E ENGENHARIA DE ALIMENTOS.
121 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
122 Zambrano, Luis, Elsa Valiente, and M. Jake Vander Zanden. 2010. Food web overlap among native axolotl (Ambystoma mexicanum) and two exotic fishes: carp (Cyprinus carpio) and tilapia (Oreochromis niloticus) in Xochimilco, Mexico City. Biological Invasions 12: 3061–3069. https://doi.org/10.1007/s10530-010-9697-8.
123 El-Sayed, A-F M. 1998. Total replacement of fish meal with animal protein sources in Nile tilapia, Oreochromis niloticus (L.), feeds. Aquaculture Research 29: 275–280. https://doi.org/10.1046/j.1365-2109.1998.00199.x.
124 El-Saidy, Deyab M. S. D., and Magdy M. A. Gaber. 2002. Complete Replacement of Fish Meal by Soybean Meal with Dietary L-Lysine Supplementation for Nile Tilapia Oreochromis niloticus (L.) Fingerlings. Journal of the World Aquaculture Society 33: 297–306. https://doi.org/10.1111/j.1749-7345.2002.tb00506.x.
125 Sharda, O. P. Sharma, and V. P. Saini. 2017. Replacement of fishmeal with soybean meal in Nile tilapia (Oreochromis niloticus) Diet. Journal of Entomology and Zoology Studies 5: 845–849.
126 Younis, El-Sayed M., Abdullah S. Al-Quffail, Nasser A. Al-Asgah, Abdel-Wahab A. Abdel-Warith, and Yousef S. Al-Hafedh. 2018. Effect of dietary fish meal replacement by red algae, Gracilaria arcuata, on growth performance and body composition of Nile tilapia Oreochromis niloticus. Saudi Journal of Biological Sciences 25: 198–203. https://doi.org/10.1016/j.sjbs.2017.06.012.
127 Khan, M. S. K., M. A. M. Siddique, and H. Zamal. 2013. Replacement of fish meal by plant protein sources in Nile tilapia (Oreochromis niloticus) diet: growth performance and utilization. Iranian Journal of Fisheries Science 12: 864–872.
128 Abdel-Warith, A.-W., N. Al-Asgah, Y. El-Sayed, A. El-Otaby, and S. Mahboob. 2018. The effect of replacement of fish meal with Amino Acids and Optimized Protein Levels in the diet of the Nile Tilapia Oreochromis niloticus. Brazilian Journal of Biology 79: 703–711. https://doi.org/10.1590/1519-6984.189413.
129 Muin, H., N. M. Taufek, M. S. Kamarudin, and S. A. Razak. 2017. Growth performance, feed Utilization and body composition of nile tilapia, Oreochromis niloticus (Linnaeus, 1758) fed with different levels of black soldier fly, Hermetia illucens (Linnaeus, 1758) maggot meal diet. Iranian Journal of Fisheries Sciences 16: 567–577.
130 El-Ouny, Youssra M., Sahya Maulu, Mohamed A. A. Zaki, Amira A. Helaly, Abdel Aziz M. Nour, Mohammed F. ElBasuini, Eman M. H. Labib, et al. 2023. Effect of fishmeal replacement with dried red wigglers (Eisenia fetida) worm meal on growth and feed utilization, production efficiency, and serum biochemistry in Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture Reports 29: 101518. https://doi.org/10.1016/j.aqrep.2023.101518.
131 Sarker, Pallab K., Anne R. Kapuscinski, Alison J. Lanois, Erin D. Livesey, Katie P. Bernhard, and Mariah L. Coley. 2016. Towards Sustainable Aquafeeds: Complete Substitution of Fish Oil with Marine Microalga Schizochytrium sp. Improves Growth and Fatty Acid Deposition in Juvenile Nile Tilapia (Oreochromis niloticus). Edited by José L Soengas. PLOS ONE 11: e0156684. https://doi.org/10.1371/journal.pone.0156684.
132 Toyes-Vargas, Eduardo A., Christopher C. Parrish, María Teresa Viana, Laura Carreón-Palau, Paola Magallón-Servín, and Francisco J. Magallón-Barajas. 2020. Replacement of fish oil with camelina (Camelina sativa) oil in diets for juvenile tilapia (var. GIFT Oreochromis niloticus) and its effect on growth, feed utilization and muscle lipid composition. Aquaculture 523: 735177. https://doi.org/10.1016/j.aquaculture.2020.735177.
133 Ayisi, Christian Larbi, Jinliang Zhao, and Jun-Wei Wu. 2018. Replacement of fish oil with palm oil: Effects on growth performance, innate immune response, antioxidant capacity and disease resistance in Nile tilapia (Oreochromis niloticus). PLOS ONE 13: e0196100. https://doi.org/10.1371/journal.pone.0196100.
134 Mood, Alison, Elena Lara, Natasha K. Boyland, and Phil Brooke. 2023. Estimating global numbers of farmed fishes killed for food annually from 1990 to 2019. Animal Welfare 32: e12. https://doi.org/10.1017/awf.2023.4.




Lorem ipsum
Something along the lines of: we were aware of the importance of some topics so that we wanted to include them and collect data but not score them. For WelfareChecks | farm, these topics are "domestication level", "feed replacement", and "commercial relevance". The domestication and commercial relevance aspects allow us to analyse the questions whether increasing rate of domestication or relevance in farming worldwide goes hand in hand with better welfare; the feed replacement rather goes in the direction of added suffering for all those species which end up as feed. For a carnivorous species, to gain 1 kg of meat, you do not just kill this one individual but you have to take into account the meat that it was fed during its life in the form of fish meal and fish oil. In other words, carnivorous species (and to a degree also omnivorous ones) have a larger "fish in:fish out" ratio.
Lorem ipsum
Probably, we updated the profile. Check the version number in the head of the page. For more information on the version, see the FAQ about this. Why do we update profiles? Not just do we want to include new research that has come out, but we are continuously developing the database itself. For example, we changed the structure of entries in criteria or we added explanations for scores in the WelfareCheck | farm. And we are always refining our scoring rules.
The centre of the Overview is an array of criteria covering basic features and behaviours of the species. Each of this information comes from our literature search on the species. If we researched a full Dossier on the species, probably all criteria in the Overview will be covered and thus filled. This was our way to go when we first set up the database.
Because Dossiers are time consuming to research, we switched to focusing on WelfareChecks. These are much shorter profiles covering just 10 criteria we deemed important when it comes to behaviour and welfare in aquaculture (and lately fisheries, too). Also, WelfareChecks contain the assessment of the welfare potential of a species which has become the main feature of the fair-fish database over time. Because WelfareChecks do not cover as many criteria as a Dossier, we don't have the information to fill all blanks in the Overview, as this information is "not investigated by us yet".
Our long-term goal is to go back to researching Dossiers for all species covered in the fair-fish database once we set up WelfareChecks for each of them. If you would like to support us financially with this, please get in touch at ffdb@fair-fish.net
See the question "What does "not investigated by us yet" mean?". In short, if we have not had a look in the literature - or in other words, if we have not investigated a criterion - we cannot know the data. If we have already checked the literature on a criterion and could not find anything, it is "no data found yet". You spotted a "no data found yet" where you know data exists? Get in touch with us at ffdb@fair-fish.net!
Once you have clicked on "show details", the entry for a criterion will unfold and display the summarised information we collected from the scientific literature – complete with the reference(s).
As reference style we chose "Springer Humanities (numeric, brackets)" which presents itself in the database as a number in a grey box. Mouse over the box to see the reference; click on it to jump to the bibliography at the bottom of the page. But what does "[x]-[y]" refer to?
This is the way we mark secondary citations. In this case, we read reference "y", but not reference "x", and cite "x" as mentioned in "y". We try to avoid citing secondary references as best as possible and instead read the original source ourselves. Sometimes we have to resort to citing secondarily, though, when the original source is: a) very old or not (digitally) available for other reasons, b) in a language no one in the team understands. Seldomly, it also happens that we are running out of time on a profile and cannot afford to read the original. As mentioned, though, we try to avoid it, as citing mistakes may always happen (and we don't want to copy the mistake) and as misunderstandings may occur by interpreting the secondarily cited information incorrectly.
If you spot a secondary reference and would like to send us the original work, please contact us at ffdb@fair-fish.net
In general, we aim at giving a good representation of the literature published on the respective species and read as much as we can. We do have a time budget on each profile, though. This is around 80-100 hours for a WelfareCheck and around 300 hours for a Dossier. It might thus be that we simply did not come around to reading the paper.
It is also possible, though, that we did have to make a decision between several papers on the same topic. If there are too many papers on one issue than we manage to read in time, we have to select a sample. On certain topics that currently attract a lot of attention, it might be beneficial to opt for the more recent papers; on other topics, especially in basic research on behaviour in the wild, the older papers might be the go-to source.
And speaking of time: the paper you are missing from the profile might have come out after the profile was published. For the publication date, please check the head of the profile at "cite this profile". We currently update profiles every 6-7 years.
If your paper slipped through the cracks and you would like us to consider it, please get in touch at ffdb@fair-fish.net
This number, for example "C | 2.1 (2022-11-02)", contains 4 parts:
- "C" marks the appearance – the design level – of the profile part. In WelfareChecks | farm, appearance "C" is our most recent one with consistent age class and label (WILD, FARM, LAB) structure across all criteria.
- "2." marks the number of major releases within this appearance. Here, it is major release 2. Major releases include e.g. changes of the WelfareScore. Even if we just add one paper – if it changes the score for one or several criteria, we will mark this as a major update for the profile. With a change to a new appearance, the major release will be re-set to 1.
- ".1" marks the number of minor updates within this appearance. Here, it is minor update 1. With minor updates, we mean changes in formatting, grammar, orthography. It can also mean adding new papers, but if these papers only confirm the score and don't change it, it will be "minor" in our book. With a change to a new appearance, the minor update will be re-set to 0.
- "(2022-11-02)" is the date of the last change – be it the initial release of the part, a minor, or a major update. The nature of the changes you may find out in the changelog next to the version number.
If an Advice, for example, has an initial release date and then just a minor update date due to link corrections, it means that – apart from correcting links – the Advice has not been updated in a major way since its initial release. Please take this into account when consulting any part of the database.
First up, you will find answers to questions for the specific page you are on. Scrolling down in the FAQ window, there are also answers to more general questions. Explore our website and the other sub pages and find there the answers to questions relevant for those pages.
In the fair-fish database, when you have chosen a species (either by searching in the search bar or in the species tree), the landing page is an Overview, introducing the most important information to know about the species that we have come across during our literatures search, including common names, images, distribution, habitat and growth characteristics, swimming aspects, reproduction, social behaviour but also handling details. To dive deeper, visit the Dossier where we collect all available ethological findings (and more) on the most important aspects during the life course, both biologically and concerning the habitat. In contrast to the Overview, we present the findings in more detail citing the scientific references.
Depending on whether the species is farmed or wild caught, you will be interested in different branches of the database.
Farm branch
Founded in 2013, the farm branch of the fair-fish database focuses on farmed aquatic species.
Catch branch
Founded in 2022, the catch branch of the fair-fish database focuses on wild-caught aquatic species.
The heart of the farm branch of the fair-fish database is the welfare assessment – or WelfareCheck | farm – resulting in the WelfareScore | farm for each species. The WelfareCheck | farm is a condensed assessment of the species' likelihood and potential for good welfare in aquaculture, based on welfare-related findings for 10 crucial criteria (home range, depth range, migration, reproduction, aggregation, aggression, substrate, stress, malformations, slaughter).
For those species with a Dossier, we conclude to-be-preferred farming conditions in the Advice | farm. They are not meant to be as detailed as a rearing manual but instead, challenge current farming standards and often take the form of what not to do.
In parallel to farm, the main element of the catch branch of the fair-fish database is the welfare assessment – or WelfareCheck | catch – with the WelfareScore | catch for each species caught with a specific catching method. The WelfareCheck | catch, too, is a condensed assessment of the species' likelihood and potential for good welfare – or better yet avoidance of decrease of good welfare – this time in fisheries. We base this on findings on welfare hazards in 10 steps along the catching process (prospection, setting, catching, emersion, release from gear, bycatch avoidance, sorting, discarding, storing, slaughter).
In contrast to the farm profiles, in the catch branch we assess the welfare separately for each method that the focus species is caught with. In the case of a species exclusively caught with one method, there will be one WelfareCheck, whereas in other species, there will be as many WelfareChecks as there are methods to catch the species with.
Summarising our findings of all WelfareChecks | catch for one species in Advice | catch, we conclude which catching method is the least welfare threatening for this species and which changes to the gear or the catching process will potentially result in improvements of welfare.
Welfare of aquatic species is at the heart of the fair-fish database. In our definition of welfare, we follow Broom (1986): “The welfare of an individual is its state as regards its attempts to cope with its environment.” Thus, welfare may be perceived as a continuum on which an individual rates “good” or “poor” or everything in between.
We pursue what could be called a combination of not only a) valuing the freedom from injuries and stress (function-based approach) but b) supporting attempts to provide rewarding experiences and cognitive challenges (feelings-based approach) as well as c) arguing for enclosures that mimic the wild habitat as best as possible and allow for natural behaviour (nature-based approach).
Try mousing over the element you are interested in - oftentimes you will find explanations this way. If not, there will be FAQ on many of the sub-pages with answers to questions that apply to the respective sub-page. If your question is not among those, contact us at ffdb@fair-fish.net.
It's right here! We decided to re-name it to fair-fish database for several reasons. The database has grown beyond dealing purely with ethology, more towards welfare in general – and so much more. Also, the partners fair-fish and FishEthoGroup decided to re-organise their partnership. While maintaining our friendship, we also desire for greater independence. So, the name "fair-fish database" establishes it as a fair-fish endeavour.