Information
Authors: Jenny Volstorf, Ana Roque
Version: B | 1.1Published: 2022-01-22
1 Remarks
1.1 General remarks
Escapees and consequences: negative or at most unpredictable for the local ecosystem1.2 Other remarks
Note of dissent:
The second author found no evidence to include L. vannamei in a group of animals which have the capacity to suffer. Nevertheless there is a growing body of literature addressing this issue in crabs which show that crabs may feel pain 5 6, have a capacity to learn 7, and have memory 8.
The editor and the first author do not agree with the insular argument to consider animal welfare only in species for which the capacity to suffer has been proven. They insist upon a wider concept of animal welfare according to the Swiss law for animal protection which respects the dignity of living beings and their integrity independent of suffering 9.
Abandoning the suffering paradigm we are able to discover other and at least as meaningful criteria for animal welfare, like e. g. joy, the opposite of pleasure. Another criterion, deception, has been investigated with the shrimp species Gonodactylus bredini with the result that the animal deceptional behaviour is functional but probably not intentional 10. Even if it was but functional, hindering a shrimp to act out its deception pattern violates its welfare. For the rest, it is obvious that the welfare of shrimps has not been a serious focus of research yet.
Even sticking to the suffering paradigm, it is likely that in shrimps, too, the capability to suffer will be proven some day 11 → FishEthoBase's understanding of fish welfare.
2 Ethograms
In the farm or lab: on feeding, daily rhythm, swimming, reproduction, stress reactions
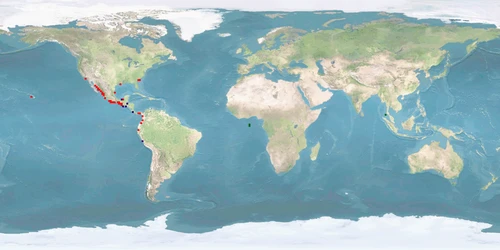
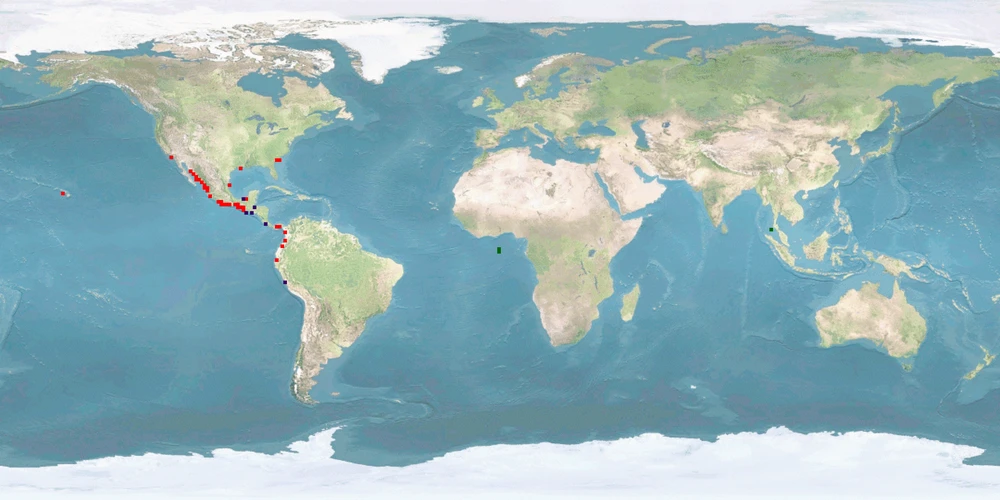
3 Distribution
Natural distribution: eastern Pacific coast
Introduced: western Atlantic coast, Asia
4 Natural co-existence
5 Substrate and/or shelter
5.1 Substrate
Substrate range, substrate preference: lives over sand and mudSubstrate and growth: mixed effects (further research needed)
5.2 Shelter or cover
Shelter or cover preference: sand for cover from the light (further research needed)6 Food, foraging, hunting, feeding
6.1 Trophic level and general considerations on food needs
Trophic level: 2.0-3.0 (further research needed)Impacts of feed fishery: contributes to overfishing, challenges animal welfare
6.2 Food items
Food items, food preference: omnivorousFeed enrichment and stress tolerance: direct effect of natural bacteria and microalgae in seawater and high proportion of highly unsaturated fatty acids (further research needed)
6.3 Feeding behaviour
Feeding style, foraging mode: depending on diet either bottom grazing or active fightFood competition and stress: related to particle size (further research needed)
Food competition and growth: unclear effect: larger individuals and males feed more often and longer but females are heavier (further research needed)
Effects on feeding: direct relation with temperature, effect of moulting cycle (further research needed)
For feeding and vision → D7.
7 Photoperiod
7.1 Daily rhythm
Daily rhythm: nocturnal (further research needed)Photoperiod and growth: mixed effects (further research needed)
7.2 Light intensity
Light intensity preference: unclear, but direct relation between light intensity and body colour darkening (further research needed)Light intensity and stress: stark light contrasts direct individuals in habitat and decrease cannibalism (further research needed)
7.3 Light colour
No data found yet.8 Water parameters
8.1 Water temperature
Standard temperature range, temperature preference: 21-37 °C, 26 °CTemperature and stress: decreasing survival <12 °C and >34-42 °C, but depends on acclimatisation and cooling or heating rate (further research needed)
Temperature and growth: optimally 28-30 °C (further research needed)
8.2 Oxygen
Dissolved oxygen range: 0.7-13.3 mg/L (further research needed)8.3 Salinity
Salinity tolerance, standard salinity range: euryhaline, 0-85 ppt depending on seasonSalinity and stress: bell-shaped relation between salinity tolerance and age, better adjusts to salinity gradually than abruptly
Salinity and growth: depends on age, water temperature, water hardness, and acclimation salinity
8.4 pH
Standard pH range: 6.0-8.0 (further research needed)8.5 Turbidity
No data found yet.8.6 Water hardness
No data found yet.8.7 NO4
No data found yet.8.8 Other
No data found yet.9 Swimming
9.1 Swimming type, swimming mode
Swimming type, swimming mode: swims with five pairs of pleopods9.2 Swimming speed
Swimming speed: 4.7 body lengths/s (juveniles at 29 °C), relatively decreasing with decreasing temperature and salinity but with increasing body length (further research needed)9.3 Home range
No data found yet.9.4 Depth
Depth range, depth preference: juveniles <1 m, adults 10-20 m (further research needed)9.5 Migration
Migration type: amphidromous10 Growth
10.1 Ontogenetic development
Mature egg: ca 13 h from fertilisation until hatching, ca 0.3 mm diameter (further research needed)Larvae: here called nauplii, protozoea, and mysis, hatching to fully shaped, 0.3-3.8 mm (further research needed)
Juveniles, sexual maturity: post-larvae (5-80 days) to beginning of maturity (6.5-8.5 months), 0.04-17.2 cm, 0.002-28.1 g
Adults: 9.5-14 months, 16.1-19.0 cm, 15.4-65 g
10.2 Sexual conversion
No data found yet.10.3 Sex ratio
No data found yet.10.4 Effects on growth
Growth rate: 0.2-1.3 mm/dGrowth and sex: bimodal pattern, noticeable from ca 6.5 months and ca 20 g on (further research needed)
Growth and other factors: direct effect of full and new moon (further research needed)
For growth and...
...substrate → D16,
...particle size → D2,
...food competition → D15,
...PHOTOPERIOD → D17,
...water temperature → D18,
...salinity → D14,
...substrate colour → D19,
...stocking density → D20.
10.5 Deformities and malformations
No data found yet.11 Reproduction
11.1 Nest building
Nest building: none11.2 Attraction, courtship, mating
Courtship sequence: male chases, turns, grasps female11.3 Spawning
Mating system: indication of promiscuity (further research needed)Male:female ratio resulting in spawning, composition of the broodstock: 1:1-6:1
Spawning sequence: male spawns at mating, female 2-5 h later (further research needed)
Effects on spawning: direct effect of wild (versus hatchery-reared) origin (further research needed)
11.4 Fecundity
Female fecundity: 30,000-80,000 eggs per mating; 62,000-219,000 eggs per mating in 1.2-2.1 matings/months when ablated (further research needed)Male fecundity: 50,000-31,900,000 sperms per spermatophore, 81,800,000 in unilaterally ablated males (further research needed)
Effects on fecundity: direct effect of wild (versus hatchery-reared) origin in females (further research needed)
Fecundity and manipulation: unilateral ablation increases female fecundity (but decreases hatching rate) and spermatophore weight and male fecundity (further research needed)
11.5 Brood care, breeding
Breeding type: sea spawner, post-larvae migrate to nursery grounds (lagoons, estuaries, mangroves)12 Senses
12.1 Vision
Visible spectrum: mainly green, lower sensitivity for violet and orange (further research needed)Light- and dark-adaptation: from 1 cm total length on, taking 30-50 s (further research needed)
Importance of vision: foraging (further research needed)
Substrate colour preference: tendency towards yellow and red (further research needed)
Substrate colour and growth: tendency towards yellow and red but might be due to contrast with food pellets (further research needed)
12.2 Olfaction (and taste, if present)
Importance of olfaction: adaptation to environment (further research needed)12.3 Hearing
No data found yet.12.4 Touch, mechanical sensing
Importance of touch: unclear (further research needed)12.5 Lateral line
No data found yet.12.6 Electrical sensing
No data found yet.12.7 Nociception, pain sensing
Nociception spectrum: recoils when eyestalk enucleated (further research needed)Pain and treatment: anaesthetic before treatment and coagulating agent afterwards decreases reactions shown otherwise (further research needed)
12.8 Other
No data found yet.13 Communication
13.1 Visual
No data found yet.13.2 Chemical
No data found yet.13.3 Acoustic
No data found yet.13.4 Mechanical
No data found yet.13.5 Electrical
No data found yet.13.6 Other
No data found yet.14 Social behaviour
14.1 Spatial organisation
Aggregation type: school (further research needed)Stocking density in the wild: 0.001-4.9 ind/m2, depending on season
Stocking density and stress: direct relation from ca 90 ind/m2 on (further research needed)
Stocking density and growth: inverse relation from ca 50 ind/m2 on (further research needed)
14.2 Social organisation
No data found yet.14.3 Exploitation
Cannibalism, predation: prevalent14.4 Facilitation
No data found yet.14.5 Aggression
For aggression and......food competition, particle size → D2,
...food competition → D29.
14.6 Territoriality
No data found yet.15 Cognitive abilities
15.1 Learning
No data found yet.15.2 Memory
No data found yet.15.3 Problem solving, creativity, planning, intelligence
No data found yet.15.4 Other
Playing: males approach, crawl under, and chase other males (further research needed)16 Personality, coping styles
17 Emotion-like states
17.1 Joy
No data found yet.17.2 Relaxation
No data found yet.17.3 Sadness
No data found yet.17.4 Fear
No data found yet.18 Self-concept, self-recognition
19 Reactions to husbandry
19.1 Stereotypical and vacuum activities
No data found yet.19.2 Acute stress
Handling: injection is stressful (further research needed)Confinement: stressful from 1 min on (further research needed)
For acute stress and water temperature → D28.
19.3 Chronic stress
Handling: stressful if repeatedly applied (further research needed)Effects on welfare: bilateral ablation increases mortality (further research needed)
For chronic stress and...
...feed enrichment → D31,
...light intensity → D3,
...water temperature → D28,
...salinity → D4,
...stocking density → D32.
19.4 Stunning reactions
Stunning rules: fast, effective, safeStunning methods: electrical stunning most effective (further research needed)
Glossary
BIOFLOC = dense microbial communities growing in flocs 31
EURYHALINE = tolerant of a wide range of salinities
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FOOD CONVERSION RATIO = (food offered / weight gained)
IND = individuals
JUVENILES = fully developed but immature individuals
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening
MILLIARD = 1,000,000,000 37 38
MYSIS = third larval stage
NAUPLII = first larval stage after hatching
PHOTOPERIOD = duration of daylight
POST-LARVAE = fully developed individuals, beginning of external sex differentiation
PROTOZOEA = second larval stage
SUB-ADULTS = juveniles transforming to fully mature adults
THELYCUM = modified region of the sternum 21
WILD = setting in the wild
Bibliography
2 Senanan, W., S. Panutrakul, P. Barnette, V. Manthachitra, S. Chavanich, A. R. Kapuscinski, N. Tangkrock-Olan, et al. 2010. Ecological risk assessemnt of an alien aquatic species: a case study of Litopenaeus vannamei (Pacific whiteleg shrimp) aquaculture in the Bangpakong river, Thailand. In Tropical Deltas and Coastal Zones: Food Production, Communities and Environment at the Land-Water Interface, ed. Chu T. Hoanh, Brian W. Szuster, Kam Suan-Pheng, Abdelbagi M. Ismail, and Andrew D. Noble, 9:64–79. Comprehensive Assessment of Water Management in Agriculture. UK: CAB International.
3 Wakida-Kusunoki, Armando T., Luis Enrique Amador-del Angel, Patricia Carrillo Alejandro, and Cecilia Quiroga Brahms. 2011. Presence of Pacific white shrimp Litopenaeus vannamei (Boone, 1931) in the Southern Gulf of Mexico. Aquatic Invasions 6: S139–S142. https://doi.org/10.3391/ai.2011.6.S1.031.
4 Panutrakul, S., W. Senanan, S. Chavanich, N. Tangkrock-Olan, and V. Viyakarn. 2010. Ability of Litopenaeus vannamei to survive and compete with local marine shrimp species in the Bangpakong river, Thailand. In Tropical Deltas and Coastal Zones: Food Production, Communities and Environment at the Land-Water Interface, ed. Chu T. Hoanh, Brian W. Szuster, Kam Suan-Pheng, Abdelbagi M. Ismail, and Andrew D. Noble, 9:80–92. Comprehensive Assessment of Water Management in Agriculture. UK: CAB International.
5 Appel, Mirjam, and Robert W. Elwood. 2009. Motivational trade-offs and potential pain experience in hermit crabs. Applied Animal Behaviour Science 119: 120–124. https://doi.org/10.1016/j.applanim.2009.03.013.
6 Barr, Stuart, Peter R. Laming, Jaimie T. A. Dick, and Robert W. Elwood. 2008. Nociception or pain in a decapod crustacean? Animal Behaviour 75: 745–751. https://doi.org/10.1016/j.anbehav.2007.07.004.
7 Elwood, RW. 2012. Evidence for pain in decapod crustaceans. Animal Welfare 21: 23–27. https://doi.org/10.7120/096272812X13353700593365.
8 Gherardi, Francesca. 2009. Behavioural indicators of pain in crustacean decapods. Annali dell’Istituto Superiore di Sanità 45: 432–438. https://doi.org/10.1590/S0021-25712009000400013.
9 Die Bundesversammlung der Schweizerischen Eidgenossenschaft. 2014. Tierschutzgesetz.
10 Kuczaj, S., K. Tranel, M. Trone, and H. Hill. 2010. Are animals capable of deception or empathy? Implications for animal consciousness and animal welfare. Animal Welfare 10: 161–173.
11 Elwood, Robert W. 2011. Pain and Suffering in Invertebrates? ILAR Journal 52: 175–184. https://doi.org/10.1093/ilar.52.2.175.
12 Moctezuma, M. A., and B. F. Blake. 1981. Burrowing Activity in Penaeus Vannamei Boone from the Caimanero-Huizache Lagoon System on the Pacific Coast of Mexico. Bulletin of Marine Science 31: 312–317.
13 Medina-Reyna, C. E. 2001. Growth and emigration of white shimp, Litopenaeus vannamei, in the Mar Muerto Lagoon, Southern Mexico. Naga, the ICLARM Quarterly 24: 30–34.
14 Rivera-Velázquez, G., L. A. Soto, I. H. Salgado-Ugarte, and E. J. Naranjo. 2008. Growth, mortality and migratory pattern of white shrimp (Litopenaeus vannamei, Crustacea, Penaeidae) in the Carretas-Pereyra coastal lagoon system, Mexico. Revista de Biología Tropical 56: 523–533.
15 Moss, Dustin R., and Shaun M. Moss. 2006. Effects of Gender and Size on Feed Acquisition in the Pacific White Shrimp Litopenaeus vannamei. Journal of the World Aquaculture Society 37: 161–167. https://doi.org/10.1111/j.1749-7345.2006.00022.x.
16 Pontes, Cibele Soares, Maria de Fatima Arruda, Alexandre Augusto de Lara Menezes, and Patrícia Pereira de Lima. 2006. Daily activity pattern of the marine shrimp Litopenaeus vannamei (Boone 1931) juveniles under laboratory conditions. Aquaculture Research 37: 1001–1006. https://doi.org/10.1111/j.1365-2109.2006.01519.x.
17 Kitani, Hiroshi. 1986. Larval Development of the White Shrimp Penaeus vannamei BOONE Reared in the Laboratory and the Statistical Observation of its Naupliar Stages. Nippon Suisan Gakkaishi 52: 1131–1139. https://doi.org/10.2331/suisan.52.1131.
18 Zhang, Peidong, Xiumei Zhang, Jian Li, and Guoqiang Huang. 2006. Swimming ability and physiological response to swimming fatigue in whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 145: 26–32. https://doi.org/10.1016/j.cbpa.2006.04.014.
19 Yu, Xiaoming, Xiumei Zhang, Yan Duan, Peidong Zhang, and Zhenqing Miao. 2010. Effects of temperature, salinity, body length, and starvation on the critical swimming speed of whiteleg shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 157: 392–397. https://doi.org/10.1016/j.cbpa.2010.08.021.
20 Yano, I., R. A. Kanna, R. N. Oyama, and J. A. Wyban. 1988. Mating behaviour in the penaeid shrimp Penaeus vannamei. Marine Biology 97: 171–175. https://doi.org/10.1007/BF00391299.
21 Misamore, Michael J., and Craig L. Browdy. 1996. Mating Behavior in the White Shrimps Penaeus setiferus and P. vannamei: A Generalized Model for Mating in Penaeus. Journal of Crustacean Biology 16: 61–70. https://doi.org/10.2307/1548931.
22 Kumlu, Metin, Serhat Türkmen, Mehmet Kumlu, and O. Tufan Eroldoğan. 2011. Off-season Maturation and Spawning of the Pacific White Shrimp Litopenaeus vannamei in Sub-tropical Conditions. Turkish Journal of Fisheries and Aquatic Sciences 11.
23 Leung-Trujillo, Joanna K., and A. L. Lawrence. 1985. The effect of eyestalk ablation on spermatophore and sperm quality in Penaeus vannamei. Journal of the World Mariculture Society 16: 258–266. https://doi.org/10.1111/j.1749-7345.1985.tb00208.x.
24 Taylor, J., L. Vinatea, R. Ozorio, R. Schuweitzer, and E. R. Andreatta. 2004. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture 233: 173–179. https://doi.org/10.1016/j.aquaculture.2003.09.034.
25 Chong-Robles, Jennyfers, Guy Charmantier, Viviane Boulo, Joel Lizárraga-Valdéz, Luis M. Enríquez-Paredes, and Ivone Giffard-Mena. 2014. Osmoregulation pattern and salinity tolerance of the white shrimp Litopenaeus vannamei (Boone, 1931) during post-embryonic development. Aquaculture 422–423: 261–267. https://doi.org/10.1016/j.aquaculture.2013.11.034.
26 Reviewed distribution maps for Pacific whitleg shrimp (Litopenaeus vannamei). 2016. Aquamaps.
27 Palacios, Elena, Ilie S. Racolta, and Acuacultores de la Paz. 1999. Spawning Frequency Analysis of Wild and Pond-Reared Pacific White Shrimp Penaeus vannamei Broodstock under Large-Scale Hatchery Conditions. Journal of the World Aquaculture Society 30: 180–191. https://doi.org/10.1111/j.1749-7345.1999.tb00865.x.
28 Rodríguez, Sergio Rendón, Emilio Macías Regalado, José Antonio Calderón Pérez, Arturo Núñez Pastén, and Rafael Solís Ibarra. 2007. Comparison of some reproductive characteristics of farmed and wild white shrimp males Litopenaeus vannamei (Decapoda: Penaeidae). International Journal of Tropical Biology and Conservation 55. https://doi.org/10.15517/rbt.v55i1.6071.
29 Briggs, M. 2006. Cultured Aquatic Species Information Programme. Penaeus vannamei. Rome: FAO Fisheries and Aquaculture Department.
30 Voltolina, Domenico, Jorge E. Watson-Toscano, Emilio Romero-Beltrán, and Juan Manuel Audelo-Naranjo. 2013. Nitrogen Recycling in Closed Cultures of Litopenaeus vannamei (Boone 1931) with Different Artificial Substrates. The Israeli Journal of Aquaculture - Bamidgeh 65.2013.907.
31 Schveitzer, Rodrigo, Rafael Arantes, Manecas Francisco Baloi, Patrícia Fóes S. Costódio, Luis Vinatea Arana, Walter Quadros Seiffert, and Edemar Roberto Andreatta. 2013. Use of artificial substrates in the culture of Litopenaeus vannamei (Biofloc System) at different stocking densities: Effects on microbial activity, water quality and production rates. Aquacultural Engineering 54: 93–103. https://doi.org/10.1016/j.aquaeng.2012.12.003.
32 Santos, Daniele Bezerra dos, Cibele Soares Pontes, Fúlvio Aurélio Morais Freire, and Ambrósio Paula Bessa Júnior. 2011. Efeito do tipo de sedimento na eficiência alimentar, crescimento e sobrevivência de Litopenaeus vannamei (Boone, 1931). Acta Scientiarum. Biological Sciences 33: 369–375. https://doi.org/10.4025/actascibiolsci.v33i4.6134.
33 FAO. 2014. The State of World Fisheries and Aquaculture 2014. Rome: Food and Agriculture Organization of the United Nations.
34 Watson, R., Jackie Alder, and Daniel Pauly. 2006. Fisheries for forage fish, 1950 to the present. In On the Multiple Uses of Forage Fish: from Ecosystems to Markets, ed. Jackie Alder and Daniel Pauly, 14:1–20. Fisheries Centre Research Reports 3. Vancouver, Canada: Fisheries Centre, University of British Columbia.
35 Mood, A. 2012. Average annual fish capture for species mostly used for fishmeal (2005-2009). fishcount.org.uk.
36 Mood, A., and P. Brooke. 2012. Estimating the Number of Farmed Fish Killed in Global Aquaculture Each Year.
37 Kopf, Von Kristin. 2012. Milliarden vs. Billionen: Große Zahlen. Sprachlog.
38 Weisstein, Eric W. 2018. Milliard. Text. MathWorld - a Wolfram Web resource. http://mathworld.wolfram.com/Milliard.html. Accessed February 2.
39 Varadharajan, D., and N. Pushparajan. 2013. Food and Feeding Habits of Aquaculture Candidate a Potential Crustacean of Pacific White Shrimp Litopenaeus Vannamei, South East Coast of India. J Aquac Res Development 4: 5. https://doi.org/10.4172/2155-9546.1000161.
40 Obaldo, Leonard G., and Reiji Masuda. 2006. Effect of Diet Size on Feeding Behavior and Growth of Pacific White Shrimp, Litopenaeus vannamei. Journal of Applied Aquaculture 18: 101–110. https://doi.org/10.1300/J028v18n01_07.
41 Mercier, Laurence, Elena Palacios, Ángel I. Campa-Córdova, Dariel Tovar-Ramírez, Roberto Hernández-Herrera, and Ilie S. Racotta. 2006. Metabolic and immune responses in Pacific whiteleg shrimp Litopenaeus vannamei exposed to a repeated handling stress. Aquaculture 258: 633–640. https://doi.org/10.1016/j.aquaculture.2006.04.036.
42 Mercier, Laurence, Ilie S. Racotta, Gloria Yepiz‐Plascencia, Adriana Muhlia‐Almazán, Roberto Civera, Marcos F. Quiñones‐Arreola, Mathieu Wille, Patrick Sorgeloos, and Elena Palacios. 2009. Effect of diets containing different levels of highly unsaturated fatty acids on physiological and immune responses in Pacific whiteleg shrimp Litopenaeus vannamei (Boone) exposed to handling stress. Aquaculture Research 40: 1849–1863. https://doi.org/10.1111/j.1365-2109.2009.02291.x.
43 Roque, Ana. 2015. Personal communication.
44 Su, Yuepeng, Shen Ma, and Cuimei Feng. 2010. Effects of Salinity Fluctuation on the Growth and Energy Budget of Juvenile Litopenaeus vannamei at Different Temperatures. Journal of Crustacean Biology 30: 430–434. https://doi.org/10.1651/09-3269.1.
45 Chan, Siu-Ming, Susan M. Rankin, and Larry L. Keeley. 1988. Characterization of the Molt Stages in Penaeus vannamei: Setogenesis and Hemolymph Levels of Total Protein, Ecdysteroids, and Glucose. The Biological Bulletin 175: 185–192.
46 You, Kui, Hongsheng Yang, Ying Liu, Shilin Liu, Yi Zhou, and Tao Zhang. 2006. Effects of different light sources and illumination methods on growth and body color of shrimp Litopenaeus vannamei. Aquaculture 252: 557–565. https://doi.org/10.1016/j.aquaculture.2005.06.041.
47 Baloi, Manecas, Rafael Arantes, Rodrigo Schveitzer, Caio Magnotti, and Luis Vinatea. 2013. Performance of Pacific white shrimp Litopenaeus vannamei raised in biofloc systems with varying levels of light exposure. Aquacultural Engineering 52: 39–44. https://doi.org/10.1016/j.aquaeng.2012.07.003.
48 Guo, Biao, Fang Wang, Ying Li, and Shuanglin Dong. 2013. Effect of periodic light intensity change on the molting frequency and growth of Litopenaeus vannamei. Aquaculture 396–399: 66–70. https://doi.org/10.1016/j.aquaculture.2013.02.033.
49 Sanudin, Noorsyarinah, Audrey Daning Tuzan, and Annita Seok Kian Yong. 2014. Feeding Activity and Growth Performance of Shrimp Post Larvae Litopenaeus vannamei Under Light and Dark Condition. Journal of Agricultural Science 6: p103. https://doi.org/10.5539/jas.v6n11p103.
50 Hsiao, Shyh-Min Tom. 2015. Method for guiding aquatic crustaceans by utilizing their biological tendency responding to bright and dark contrast. http://www.google.com/patents/US7000567. Accessed November 12.
51 González, Ricardo A., Fernando Díaz, Alexei Licea, Ana Denisse Re, L. Noemí Sánchez, and Zaul García-Esquivel. 2010. Thermal preference, tolerance and oxygen consumption of adult white shrimp Litopenaeus vannamei (Boone) exposed to different acclimation temperatures. Journal of Thermal Biology 35: 218–224. https://doi.org/10.1016/j.jtherbio.2010.05.004.
52 Kumlu, Metin, Serhat Türkmen, and Mehmet Kumlu. 2010. Thermal tolerance of Litopenaeus vannamei (Crustacea: Penaeidae) acclimated to four temperatures. Journal of Thermal Biology 35: 305–308. https://doi.org/10.1016/j.jtherbio.2010.06.009.
53 Ogle, John T., Kathy Beaugez, and Jeffrey M. Lotz. 1992. Effects of Salinity on Survival and Growth of Postlarval Penaeus vannamei. Gulf and Caribbean Research 8: 415–421. https://doi.org/10.18785/grr.0804.07.
54 Perez-Velazquez, Martin, Mayra L. González-Félix, D. A. Davis, Luke A. Roy, and Xuezhi Zhu. 2013. Studies of the Thermal and Haline Influences on Growth and Survival of Litopenaeus vannamei and Litopenaeus setiferus. Journal of the World Aquaculture Society 44: 229–238. https://doi.org/10.1111/jwas.12028.
55 McGraw, W. J., D. A. Davis, D. Teichert-Coddington, and D. B. Rouse. 2002. Acclimation of Litopenaeus vannamei Postlarvae to Low Salinity: Influence of Age, Salinity Endpoint, and Rate of Salinity Reduction. Journal of the World Aquaculture Society 33: 78–84.
56 Jayasankar, Vidya, Safiah Jasmani, Takeshi Nomura, Setsuo Nohara, Do Thi Thanh Huong, and Marcy N. Wilder. 2009. Low Salinity Rearing of the Pacific White Shrimp Litopenaeus vannamei: Acclimation, Survival and Growth of Postlarvae and Juveniles. Japan Agricultural Research Quarterly: JARQ 43: 345–350. https://doi.org/10.6090/jarq.43.345.
57 Parnes, S, E Mills, C Segall, S Raviv, C Davis, and A Sagi. 2004. Reproductive readiness of the shrimp Litopenaeus vannamei grown in a brackish water system. Aquaculture 236: 593–606. https://doi.org/10.1016/j.aquaculture.2004.01.040.
58 Sainz-Hernández, Juan Carlos, Ilie S. Racotta, Silvie Dumas, and Jorge Hernández-López. 2008. Effect of unilateral and bilateral eyestalk ablation in Litopenaeus vannamei male and female on several metabolic and immunologic variables. Aquaculture 283: 188–193. https://doi.org/10.1016/j.aquaculture.2008.07.002.
59 Garza-Torres, Rodolfo, Rafael Campos-Ramos, and Alejandro M. Maeda-Martínez. 2009. Organogenesis and subsequent development of the genital organs in female and male Pacific white shrimp Penaeus (Litopenaeus) vannamei. Aquaculture 296: 136–142. https://doi.org/10.1016/j.aquaculture.2009.08.012.
60 Wasielesky, Wilson, Charles Froes, Geraldo Fóes, Dariano Krummenauer, Gabriele Lara, and Luis Poersch. 2013. Nursery of Litopenaeus vannamei Reared in a Biofloc System: The Effect of Stocking Densities and Compensatory Growth. Journal of Shellfish Research 32: 799–806. https://doi.org/10.2983/035.032.0323.
61 Otoshi, Clete A., Scott S. Naguwa, Frank C. Falesch, and Shaun M. Moss. 2007. Shrimp behavior may affect culture performance at super-intensive stocking densities. Global Aquaculture Advocate March/April: 67–69.
62 Sookying, Daranee, Fabio Soller D. Silva, D. Allen Davis, and Terrill R. Hanson. 2011. Effects of stocking density on the performance of Pacific white shrimp Litopenaeus vannamei cultured under pond and outdoor tank conditions using a high soybean meal diet. Aquaculture 319: 232–239. https://doi.org/10.1016/j.aquaculture.2011.06.014.
63 Aparicio-Simón, Benjamin, Manuel Piñón, Radu Racotta, and Ilie S. Racotta. 2010. Neuroendocrine and metabolic responses of Pacific whiteleg shrimp Litopenaeus vannamei exposed to acute handling stress. Aquaculture 298: 308–314. https://doi.org/10.1016/j.aquaculture.2009.10.016.
64 Matsuda, Keishi, and Marcy N. Wilder. 2010. Difference in light perception capability and spectral response between juveniles and sub-adults of the whiteleg shrimp Litopenaeus vannamei as determined by electroretinogram. Fisheries Science 76: 633–641. https://doi.org/10.1007/s12562-010-0253-3.
65 Wong, Enrique. 2014. Personal communication.
66 Ammar, Dib, Evelise Maria Nazari, Yara Maria Rauh Müller, and Silvana Allodi. 2008. New Insights on the Olfactory Lobe of Decapod Crustaceans. Brain, Behavior and Evolution 72: 27–36. https://doi.org/10.1159/000139459.
67 Griffith, D. R. W., and J. M. Wigglesworth. 1993. Growth rhythms in the shrimp Penaeus vannamei and P. schmitti. Marine Biology 115: 295–299. https://doi.org/10.1007/BF00346347.
68 Luchiari, A. C., A. O. Marques, and F. A. M. Freire. 2012. Effects of substrate colour preference on growth of the shrimp Litopenaeus vannamei (Boone, 1931) (Decapoda, Penaeoidea). Crustaceana 85: 789–800. https://doi.org/10.1163/156854012X650232.
69 Schmidt, M., and S. Harzsch. 1999. Comparative Analysis of Neurogenesis in the Central Olfactory Pathway of Adult Decapod Crustaceans by In Vivo BrdU Labeling. The Biological Bulletin 196: 127–136.
70 Vickery, Rachel, Kathleen Hollowell, and Melissa Hughes. 2012. Why have long antennae? Exploring the function of antennal contact in snapping shrimp. Marine and Freshwater Behaviour and Physiology 45: 161–176. https://doi.org/10.1080/10236244.2012.699644.
71 Balakrishnan, Gunalan, Soundarapandian Peyail, Kumaran Ramachandran, Anand Theivasigamani, Maheswaran Chokkaiah, and Pushparaj Nataraj. 2011. Growth of Cultured White Leg Shrimp Litopenaeus Vannamei (Boone 1931) In Different Stocking Density. Advances in Applied Science Research 2: 107–113.
72 Robb, D H F, and S C Kestin. 2002. Methods Used to Kill Fish: Field Observations and Literature Reviewed. Animal Welfare 11: 269–282.
73 Roth, B, and S Øines. 2010. Stunning and killing of edible crabs (Cancer pagurus). Animal Welfare 19: 287–294.
74 Sparrey, Julian. 2005. Testing of Crustastun single crab and lobster stunner. Unpublished research report. UK.
75 Neil, Douglas. 2010. The effect of the CrustastunTM on nerve activity in crabs and lobsters. Scientific Report to Studham Technologies Ltd. UK: University of Glasgow.
76 Neil, Douglas, and John Thompson. 2012. The Stress Induced by the CrustastunTM Process in Two Commercially Important Decapod Crustaceans: The Edible Brown Cancer Pagurus and the European Lobster Homarus Gammarus. Project report. UK: University of Glasgow.
77 Bickmeyer, Ulf, and Torsten Fregin. 2015. Vergleichende Untersuchungen zur tiergerechten Betäubung oder Tötung von Krustentieren. Bremerhaven: Alfred Wegener Institut, Helmholtz Zentrum für Polar- und Meeresforschung.





Probably, we updated the profile. Check the version number in the head of the page. For more information on the version, see the FAQ about this. Why do we update profiles? Not just do we want to include new research that has come out, but we are continuously developing the database itself. For example, we changed the structure of entries in criteria or we added explanations for scores in the WelfareCheck | farm. And we are always refining our scoring rules.
The centre of the Overview is an array of criteria covering basic features and behaviours of the species. Each of this information comes from our literature search on the species. If we researched a full Dossier on the species, probably all criteria in the Overview will be covered and thus filled. This was our way to go when we first set up the database.
Because Dossiers are time consuming to research, we switched to focusing on WelfareChecks. These are much shorter profiles covering just 10 criteria we deemed important when it comes to behaviour and welfare in aquaculture (and lately fisheries, too). Also, WelfareChecks contain the assessment of the welfare potential of a species which has become the main feature of the fair-fish database over time. Because WelfareChecks do not cover as many criteria as a Dossier, we don't have the information to fill all blanks in the Overview, as this information is "not investigated by us yet".
Our long-term goal is to go back to researching Dossiers for all species covered in the fair-fish database once we set up WelfareChecks for each of them. If you would like to support us financially with this, please get in touch at ffdb@fair-fish.net
See the question "What does "not investigated by us yet" mean?". In short, if we have not had a look in the literature - or in other words, if we have not investigated a criterion - we cannot know the data. If we have already checked the literature on a criterion and could not find anything, it is "no data found yet". You spotted a "no data found yet" where you know data exists? Get in touch with us at ffdb@fair-fish.net!
Once you have clicked on "show details", the entry for a criterion will unfold and display the summarised information we collected from the scientific literature – complete with the reference(s).
As reference style we chose "Springer Humanities (numeric, brackets)" which presents itself in the database as a number in a grey box. Mouse over the box to see the reference; click on it to jump to the bibliography at the bottom of the page. But what does "[x]-[y]" refer to?
This is the way we mark secondary citations. In this case, we read reference "y", but not reference "x", and cite "x" as mentioned in "y". We try to avoid citing secondary references as best as possible and instead read the original source ourselves. Sometimes we have to resort to citing secondarily, though, when the original source is: a) very old or not (digitally) available for other reasons, b) in a language no one in the team understands. Seldomly, it also happens that we are running out of time on a profile and cannot afford to read the original. As mentioned, though, we try to avoid it, as citing mistakes may always happen (and we don't want to copy the mistake) and as misunderstandings may occur by interpreting the secondarily cited information incorrectly.
If you spot a secondary reference and would like to send us the original work, please contact us at ffdb@fair-fish.net
In general, we aim at giving a good representation of the literature published on the respective species and read as much as we can. We do have a time budget on each profile, though. This is around 80-100 hours for a WelfareCheck and around 300 hours for a Dossier. It might thus be that we simply did not come around to reading the paper.
It is also possible, though, that we did have to make a decision between several papers on the same topic. If there are too many papers on one issue than we manage to read in time, we have to select a sample. On certain topics that currently attract a lot of attention, it might be beneficial to opt for the more recent papers; on other topics, especially in basic research on behaviour in the wild, the older papers might be the go-to source.
And speaking of time: the paper you are missing from the profile might have come out after the profile was published. For the publication date, please check the head of the profile at "cite this profile". We currently update profiles every 6-7 years.
If your paper slipped through the cracks and you would like us to consider it, please get in touch at ffdb@fair-fish.net
This number, for example "C | 2.1 (2022-11-02)", contains 4 parts:
- "C" marks the appearance – the design level – of the profile part. In WelfareChecks | farm, appearance "C" is our most recent one with consistent age class and label (WILD, FARM, LAB) structure across all criteria.
- "2." marks the number of major releases within this appearance. Here, it is major release 2. Major releases include e.g. changes of the WelfareScore. Even if we just add one paper – if it changes the score for one or several criteria, we will mark this as a major update for the profile. With a change to a new appearance, the major release will be re-set to 1.
- ".1" marks the number of minor updates within this appearance. Here, it is minor update 1. With minor updates, we mean changes in formatting, grammar, orthography. It can also mean adding new papers, but if these papers only confirm the score and don't change it, it will be "minor" in our book. With a change to a new appearance, the minor update will be re-set to 0.
- "(2022-11-02)" is the date of the last change – be it the initial release of the part, a minor, or a major update. The nature of the changes you may find out in the changelog next to the version number.
If an Advice, for example, has an initial release date and then just a minor update date due to link corrections, it means that – apart from correcting links – the Advice has not been updated in a major way since its initial release. Please take this into account when consulting any part of the database.
First up, you will find answers to questions for the specific page you are on. Scrolling down in the FAQ window, there are also answers to more general questions. Explore our website and the other sub pages and find there the answers to questions relevant for those pages.
In the fair-fish database, when you have chosen a species (either by searching in the search bar or in the species tree), the landing page is an Overview, introducing the most important information to know about the species that we have come across during our literatures search, including common names, images, distribution, habitat and growth characteristics, swimming aspects, reproduction, social behaviour but also handling details. To dive deeper, visit the Dossier where we collect all available ethological findings (and more) on the most important aspects during the life course, both biologically and concerning the habitat. In contrast to the Overview, we present the findings in more detail citing the scientific references.
Depending on whether the species is farmed or wild caught, you will be interested in different branches of the database.
Farm branch
Founded in 2013, the farm branch of the fair-fish database focuses on farmed aquatic species.
Catch branch
Founded in 2022, the catch branch of the fair-fish database focuses on wild-caught aquatic species.
The heart of the farm branch of the fair-fish database is the welfare assessment – or WelfareCheck | farm – resulting in the WelfareScore | farm for each species. The WelfareCheck | farm is a condensed assessment of the species' likelihood and potential for good welfare in aquaculture, based on welfare-related findings for 10 crucial criteria (home range, depth range, migration, reproduction, aggregation, aggression, substrate, stress, malformations, slaughter).
For those species with a Dossier, we conclude to-be-preferred farming conditions in the Advice | farm. They are not meant to be as detailed as a rearing manual but instead, challenge current farming standards and often take the form of what not to do.
In parallel to farm, the main element of the catch branch of the fair-fish database is the welfare assessment – or WelfareCheck | catch – with the WelfareScore | catch for each species caught with a specific catching method. The WelfareCheck | catch, too, is a condensed assessment of the species' likelihood and potential for good welfare – or better yet avoidance of decrease of good welfare – this time in fisheries. We base this on findings on welfare hazards in 10 steps along the catching process (prospection, setting, catching, emersion, release from gear, bycatch avoidance, sorting, discarding, storing, slaughter).
In contrast to the farm profiles, in the catch branch we assess the welfare separately for each method that the focus species is caught with. In the case of a species exclusively caught with one method, there will be one WelfareCheck, whereas in other species, there will be as many WelfareChecks as there are methods to catch the species with.
Summarising our findings of all WelfareChecks | catch for one species in Advice | catch, we conclude which catching method is the least welfare threatening for this species and which changes to the gear or the catching process will potentially result in improvements of welfare.
Welfare of aquatic species is at the heart of the fair-fish database. In our definition of welfare, we follow Broom (1986): “The welfare of an individual is its state as regards its attempts to cope with its environment.” Thus, welfare may be perceived as a continuum on which an individual rates “good” or “poor” or everything in between.
We pursue what could be called a combination of not only a) valuing the freedom from injuries and stress (function-based approach) but b) supporting attempts to provide rewarding experiences and cognitive challenges (feelings-based approach) as well as c) arguing for enclosures that mimic the wild habitat as best as possible and allow for natural behaviour (nature-based approach).
Try mousing over the element you are interested in - oftentimes you will find explanations this way. If not, there will be FAQ on many of the sub-pages with answers to questions that apply to the respective sub-page. If your question is not among those, contact us at ffdb@fair-fish.net.
It's right here! We decided to re-name it to fair-fish database for several reasons. The database has grown beyond dealing purely with ethology, more towards welfare in general – and so much more. Also, the partners fair-fish and FishEthoGroup decided to re-organise their partnership. While maintaining our friendship, we also desire for greater independence. So, the name "fair-fish database" establishes it as a fair-fish endeavour.
