Information
Author: Jenny Volstorf
Version: B | 1.1Published: 2022-10-05
1 Remarks
1.1 General remarks
Escapees and consequences: negative or at most unpredictable for the local ecosystem1.2 Other remarks
No data found yet.2 Ethograms
In the farm or lab: on daily rhythm, social behaviour, cognitive abilities, coping styles
3 Distribution
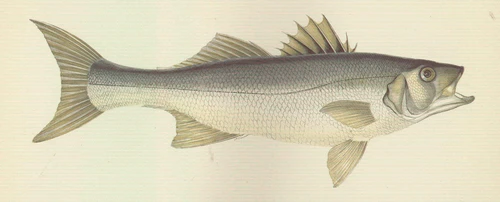
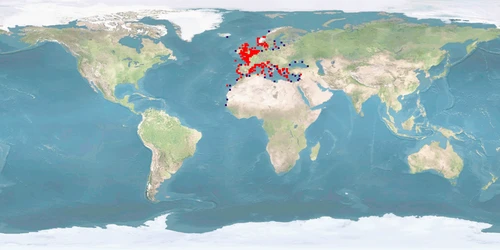
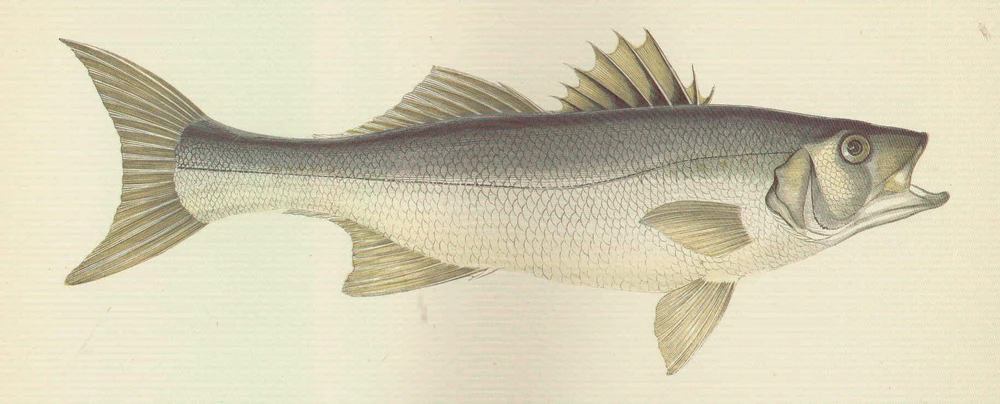
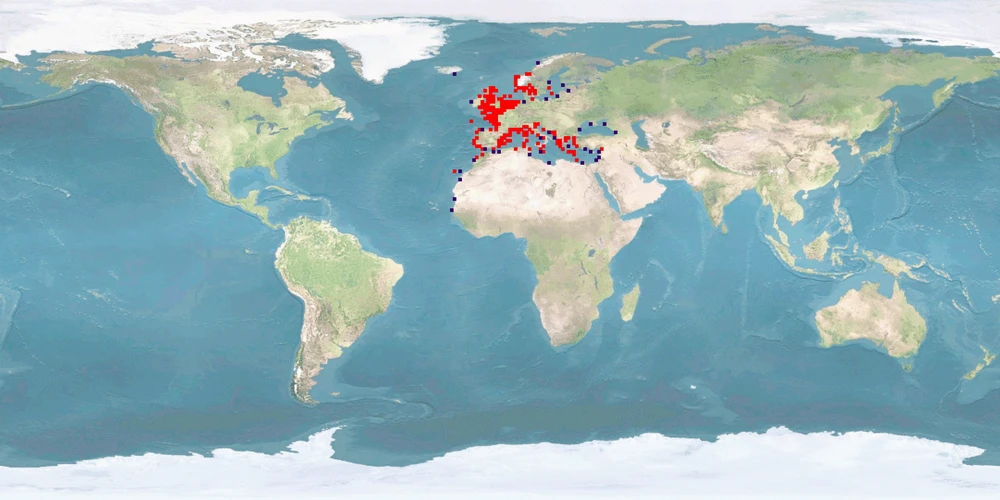
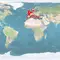
Natural distribution: Mediterranean, eastern Atlantic, British Isles
4 Natural co-existence
5 Substrate and/or shelter
5.1 Substrate
Substrate range, substrate preference: opportunistic – reported from coasts with plants as well as with sandy or muddy bottoms5.2 Shelter or cover
No data found yet.6 Food, foraging, hunting, feeding
6.1 Trophic level and general considerations on food needs
Trophic level: 3-4.6, increasing with body sizeImpacts of feed fishery: contributes to overfishing, challenges animal welfare
6.2 Food items
Food items, food preference: carnivorous, increasing prey size with increasing age6.3 Feeding behaviour
Feeding style, foraging mode: depending on diet either bottom grazing or active pursuitFeed delivery and stress: unpredicted schedule increases stress and swimming activity
For feeding and...
...social structure → D4,
...shyness-boldness continuum → D5,
...exploration-avoidance continuum → D6.
7 Photoperiod
7.1 Daily rhythm
Daily rhythm: diurnal7.2 Light intensity
No data found yet.7.3 Light colour
No data found yet.8 Water parameters
8.1 Water temperature
Standard temperature range, temperature preference: 10-27 °CTemperature and stress: lower survival and higher stress <20 °C, repeatedly switching temperature is stressful (further research needed)
Temperature and growth: must exceed 13-14 °C, optimal at 18-26 °C (further research needed)
8.2 Oxygen
Dissolved oxygen range: 4.5-12 mg/L (further research needed)8.3 Salinity
Salinity tolerance, standard salinity range: euryhaline, 0-35 ppt8.4 pH
No data found yet.8.5 Turbidity
No data found yet.8.6 Water hardness
No data found yet.8.7 NO4
No data found yet.8.8 Other
No data found yet.9 Swimming
9.1 Swimming type, swimming mode
Swimming type, swimming mode: sub-carangiform9.2 Swimming speed
Swimming speed: 3.6-4.1 body lengths/s, relatively decreasing with body length, depending on acclimation temperature (further research needed)Standard velocity range, velocity preference: 0-200+ cm/min (further research needed)
9.3 Home range
Home range: 0.6-16 km9.4 Depth
Depth range, depth preference: 0.8-73 m9.5 Migration
Migration type: amphidromous10 Growth
10.1 Ontogenetic development
Mature egg: 4 days from fertilisation until hatching, 1.2-1.4 mm diameter (further research needed)Larvae: hatching to 9 days, 4-6 mm (further research needed)
Fry: beginning of exogenous feeding, 9-365 days, 3-17 cm, 0.3-8.5 g
Juveniles, sexual maturity: fully developed (ca 4-12 months) to beginning of maturity (5-7 years), 6-42 cm, 8-639.4 g
Adults: 2-24 years, 18-87.8 cm, 230-6,900 g
10.2 Sexual conversion
Sex and manipulation: androgen treatment increases portion of male fry, estrogen treatment portion of female fry (further research needed)10.3 Sex ratio
Natural male:female ratio: 1:2 (further research needed)10.4 Effects on growth
Growth rate: 7.4-9.6 cm/year in first year (peak 1.5 mm/d), 2.1-3.2 cm/year from eigth year onGrowth and sex: bimodal pattern, noticeable from 10 months on (further research needed)
Growth and size-grading: no effect (further research needed)
For growth and...
...temperature → D13,
...stocking density → D14.
10.5 Deformities and malformations
No data found yet.11 Reproduction
11.1 Nest building
Nest building: none11.2 Attraction, courtship, mating
No data found yet.11.3 Spawning
Spawning conditions: no substrate, winter-spring, 10-15 °C, 14-35 pptMale:female ratio resulting in spawning, composition of the broodstock: 1:1-1:3 (further research needed)
Spawning sequence: female releases eggs in batches over longer time (further research needed)
11.4 Fecundity
Female fecundity: 293,000-358,000 eggs/kg body weight (further research needed)11.5 Brood care, breeding
Breeding type: sea spawner, larvae migrate to nursery grounds (lagoons, estuaries, river mouths)12 Senses
12.1 Vision
No data found yet.12.2 Olfaction (and taste, if present)
No data found yet.12.3 Hearing
Hearing type, hearing spectrum: hearing generalist12.4 Touch, mechanical sensing
No data found yet.12.5 Lateral line
No data found yet.12.6 Electrical sensing
No data found yet.12.7 Nociception, pain sensing
No data found yet.12.8 Other
No data found yet.13 Communication
13.1 Visual
No data found yet.13.2 Chemical
No data found yet.13.3 Acoustic
No data found yet.13.4 Mechanical
No data found yet.13.5 Electrical
No data found yet.13.6 Other
No data found yet.14 Social behaviour
14.1 Spatial organisation
Aggregation type: juveniles and adults in shoals or schools, adults also solitary (further research needed)Stocking density in the wild: 0.4-1.0 ind/1,000 m2, max 81 ind/1,000 m2 (further research needed)
Stocking density and stress: direct relation from ca 20 kg/m3 on, tolerates more on short term but stress increases on long term (further research needed)
Stocking density and growth: mixed effects (further research needed)
14.2 Social organisation
Social organisation type: hardly establish linear hierarchy (when in small groups), but in habitat with self-feeder, few individuals trigger majority of feed (further research needed)14.3 Exploitation
No data found yet.14.4 Facilitation
Cooperation, mutualism: shoal to pursue prey (further research needed)14.5 Aggression
Aggression and stocking density: no effect (further research needed)Aggression and size-grading: no effect because hardly aggressive (further research needed)
14.6 Territoriality
Territoriality and feeding: no relation (further research needed)15 Cognitive abilities
15.1 Learning
Operant or instrumental conditioning: may be used for managing self-feeder15.2 Memory
No data found yet.15.3 Problem solving, creativity, planning, intelligence
No data found yet.15.4 Other
No data found yet.16 Personality, coping styles
Exploration-avoidance continuum: relationship with feeding recovery (further research needed)
Aggressiveness continuum: given stocking density and size-grading
17 Emotion-like states
17.1 Joy
No data found yet.17.2 Relaxation
No data found yet.17.3 Sadness
No data found yet.17.4 Fear
No data found yet.18 Self-concept, self-recognition
19 Reactions to husbandry
19.1 Stereotypical and vacuum activities
No data found yet.19.2 Acute stress
Confinement: stressful if done for 1 h (further research needed)Crowding: stressful if done at 50 kg/m3 for 10 min (further research needed)
For acute stress and...
...stunning → D21.
19.3 Chronic stress
Effects on welfare: cage submergence may be beneficial (further research needed)For chronic stress and...
...feed delivery → D22,
...temperature → D17,
...stocking density → D23,
...social organisation type → D4.
19.4 Stunning reactions
Stunning rules: fast, effective, safeStunning methods: electrical stunning most effective (further research needed)
Stunning methods and stress: lowest struggle time and stress at combination of clove oil anaesthesia and ice-water slurry hypothermia, at absence of crowding (further research needed)
Glossary
AGGRESSIVENESS = agonistic reactions towards conspecifics. Tests: mirror image, social interaction/diadic encounters 51.
EXPLORATION-AVOIDANCE = reaction to new situations, e.g. new habitat, new food, novel objects. Referred to as neophobia/neophilia elsewhere. Tests: open field, trappability for first time, novel environment, hole board (time spent with head in holes), novel object 51.
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FISHES = using "fishes" instead of "fish" for more than one individual - whether of the same species or not - is inspired by Jonathan Balcombe who proposed this usage in his book "What a fish knows". By referring to a group as "fishes", we acknowledge the individuals with their personalities and needs instead of an anonymous mass of "fish".
FOOD CONVERSION RATIO = (food offered / weight gained)
FRY = larvae from external feeding on
GENERALIST = Generalists detect a narrow bandwidth of sound frequencies (<50-500 Hz, 1,500 Hz max.). High hearing threshold = cannot detect quieter sounds. Typically no swim bladder or no attachment of the swim bladder to the inner ear. Live in loud environments (rivers) 4849.
IND = individuals
JUVENILES = fully developed but immature individuals
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening
MILLIARD = 1,000,000,000 31 32
PHOTOPERIOD = duration of daylight
POST-LARVAE = fully developed individuals, beginning of external sex differentiation
SHYNESS-BOLDNESS = reaction to risky (but not new!) situations, e.g. predators or humans. Referred to as docility, tameness, fearfulness elsewhere. Tests: predator presentation, predator stimulus, threat, trappability (latency to enter a trap for first time can be exploration), resistance to handlers (Trapezov stick test), tonic immobility (catatonic-like death-feigning anti predator response) 51.
TOTAL LENGTH = from snout to tip of caudal fin as compared to fork length (which measures from snout to fork of caudal fin) or standard length (from head to base of tail fin) or body length (from the base of the eye notch to the posterior end of the telson) 23
WILD = setting in the wild
Bibliography
2 Bégout Anras, M-L, J-P Lagardére, and J-Y Lafaye. 1997. Diel activity rhythm of seabass tracked in a natural environment: group effects on swimming patterns and amplitudes. Canadian Journal of Fisheries and Aquatic Sciences 54: 162–168. https://doi.org/10.1139/f96-253.
3 Kennedy, Michael, and Patrick Fitzmaurice. 1972. The Biology of the Bass, Dicentrarchus Labrax, in Irish Waters. Journal of the Marine Biological Association of the United Kingdom 52: 557. https://doi.org/10.1017/S0025315400021597.
4 Holden, M. J., and T. Williams. 1974. The Biology, Movements and Population Dynamics of Bass, Dicentrarchus Labrax, in English Waters. Journal of the Marine Biological Association of the United Kingdom 54: 91. https://doi.org/10.1017/S0025315400022098.
5 Brehmer, Patrice, Thang Do Chi, and David Mouillot. 2006. Amphidromous fish school migration revealed by combining fixed sonar monitoring (horizontal beaming) with fishing data. Journal of Experimental Marine Biology and Ecology 334: 139–150. https://doi.org/10.1016/j.jembe.2006.01.017.
6 Martinho, F., R. Leitão, J. M. Neto, H. Cabral, F. Lagardère, and M. A. Pardal. 2008. Estuarine colonization, population structure and nursery functioning for 0-group sea bass (Dicentrarchus labrax), flounder (Platichthys flesus) and sole (Solea solea) in a mesotidal temperate estuary. Journal of Applied Ichthyology 24: 229–237. https://doi.org/10.1111/j.1439-0426.2007.01049.x.
7 Benhaïm, David, Marie-Laure Bégout, Gaël Lucas, and Béatrice Chatain. 2013. First Insight into Exploration and Cognition in Wild Caught and Domesticated Sea Bass ( Dicentrarchus labrax ) in a Maze. PLOS ONE 8: e65872. https://doi.org/10.1371/journal.pone.0065872.
8 Benhaïm, David, Samuel Péan, Blandine Brisset, Didier Leguay, Marie-Laure Bégout, and Béatrice Chatain. 2011. Effect of size grading on sea bass (Dicentrarchus labrax) juvenile self-feeding behaviour, social structure and culture performance. Aquatic Living Resources 24: 391–402. https://doi.org/10.1051/alr/2011140.
9 Ferrari, Sébastien, David Benhaïm, Tatiana Colchen, Béatrice Chatain, and Marie-Laure Bégout. 2014. First links between self-feeding behaviour and personality traits in European seabass, Dicentrarchus labrax. Applied Animal Behaviour Science 161: 131–141. https://doi.org/10.1016/j.applanim.2014.09.019.
10 Carbonara, P., M. Scolamacchia, M. T. Spedicato, G. Lembo, W. Zupa, and R. S. McKinley. 2006. Swimming performance as a well-being indicator of reared sea-bass Dicentrarchus labrax (Linnaeus, 1758). Preliminary results. Biol. Mar. Medit. 13: 488–491.
11 Claireaux, Guy, Christine Couturier, and Anne-Laure Groison. 2006. Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). Journal of Experimental Biology 209: 3420–3428. https://doi.org/10.1242/jeb.02346.
12 Koumoundouros, G., C. Ashton, D. G. Sfakianakis, P. Divanach, M. Kentouri, N. Anthwal, and N. C. Stickland. 2009. Thermally induced phenotypic plasticity of swimming performance in European sea bass Dicentrarchus labrax juveniles. Journal of Fish Biology 74: 1309–1322. https://doi.org/10.1111/j.1095-8649.2009.02206.x.
13 Marras, S., G. Claireaux, D. J. McKenzie, and J. A. Nelson. 2010. Individual variation and repeatability in aerobic and anaerobic swimming performance of European sea bass, Dicentrarchus labrax. Journal of Experimental Biology 213: 26–32. https://doi.org/10.1242/jeb.032136.
14 Di-Poï, C., J. Attia, C. Bouchut, G. Dutto, D. Covès, and M. Beauchaud. 2007. Behavioral and neurophysiological responses of European sea bass groups reared under food constraint. Physiology & Behavior 90: 559–566. https://doi.org/10.1016/j.physbeh.2006.11.005.
15 Ferrari, Sébastien, Sandie Millot, Didier Leguay, Béatrice Chatain, and Marie-Laure Bégout. 2015. Consistency in European seabass coping styles: A life-history approach. Applied Animal Behaviour Science 167: 74–88. https://doi.org/10.1016/j.applanim.2015.03.006.
16 Lupatsch, I., G.A. Santos, J.W. Schrama, and J.A.J. Verreth. 2010. Effect of stocking density and feeding level on energy expenditure and stress responsiveness in European sea bass Dicentrarchus labrax. Aquaculture 298: 245–250. https://doi.org/10.1016/j.aquaculture.2009.11.007.
17 Benhaïm, David, Samuel Péan, Gaël Lucas, Nancy Blanc, Béatrice Chatain, and Marie-Laure Bégout. 2012. Early life behavioural differences in wild caught and domesticated sea bass (Dicentrarchus labrax). Applied Animal Behaviour Science 141: 79–90. https://doi.org/10.1016/j.applanim.2012.07.002.
18 Reviewed distribution maps for European seabass (Dicentrarchus labrax). 2016. Aquamaps.
19 Rogdakis, Yiannis, Alexis Ramfos, Katerina Koukou, Evagelos Dimitriou, and George Katselis. 2010. Feeding habits and trophic level of sea bass (Dicentrarchus labrax) in the Messolonghi-Etoliko lagoons complex (Western Greece). Journal of Biological Research 13: 13–26.
20 Vinagre, C., T. Ferreira, L. Matos, M. J. Costa, and H. N. Cabral. 2009. Latitudinal gradients in growth and spawning of sea bass, Dicentrarchus labrax, and their relationship with temperature and photoperiod. Estuarine, Coastal and Shelf Science 81: 375–380. https://doi.org/10.1016/j.ecss.2008.11.015.
21 Cabral, Henrique, and Maria José Costa. 2001. Abundance, feeding ecology and growth of 0-group sea bass, Dicentrarchus labrax, within the nursery areas of the Tagus estuary. Journal of the Marine Biological Association of the United Kingdom 81: 679–682. https://doi.org/10.1017/S0025315401004362.
22 Pickett, G. D. 1990. Assessment of the UK bass fishery using a log-book-based catch recording system. Fisheries Research Technical Report 90. Lowestoft: Directorate of Fisheries Research.
23 Pawson, M.G., and G.D. Pickett. 1996. The Annual Pattern of Condition and Maturity in Bass, Dicentrarchus Labrax, in Waters Around England and Wales. Journal of the Marine Biological Association of the United Kingdom 76: 107. https://doi.org/10.1017/S0025315400029040.
24 Claridge, P. N., and I. C. Potter. 1983. Movements, abundance, age composition and growth of bass, Dicentrarchus labrax, in the Severn Estuary and inner Bristol Channel. Journal of the Marine Biological Association of the United Kingdom 63: 871–879. https://doi.org/10.1017/S0025315400071289.
25 Fahy, E., N. Forrest, U. Shaw, and P. Green. 2000. Observations on the status of bass Dicentrarchus Labrax stocks in Ireland in the late 1990s. Irish Fisheries Investigations 5.
26 Froese, R., and D. Pauly. 2014. FishBase. World Wide Web electronic publication. www.fishbase.org.
27 FAO. 2014. The State of World Fisheries and Aquaculture 2014. Rome: Food and Agriculture Organization of the United Nations.
28 Watson, R., Jackie Alder, and Daniel Pauly. 2006. Fisheries for forage fish, 1950 to the present. In On the Multiple Uses of Forage Fish: from Ecosystems to Markets, ed. Jackie Alder and Daniel Pauly, 14:1–20. Fisheries Centre Research Reports 3. Vancouver, Canada: Fisheries Centre, University of British Columbia.
29 Mood, A. 2012. Average annual fish capture for species mostly used for fishmeal (2005-2009). fishcount.org.uk.
30 Mood, A., and P. Brooke. 2012. Estimating the Number of Farmed Fish Killed in Global Aquaculture Each Year.
31 Kopf, Von Kristin. 2012. Milliarden vs. Billionen: Große Zahlen. Sprachlog.
32 Weisstein, Eric W. 2018. Milliard. Text. MathWorld - a Wolfram Web resource. http://mathworld.wolfram.com/Milliard.html. Accessed February 2.
33 Sarà, G., A. Oliveri, G. Martino, S. Serra, G. Meloni, and A. Pais. 2010. Response of captive seabass and seabream as behavioural indicator in aquaculture. Italian Journal of Animal Science 6: 823–825. https://doi.org/10.4081/ijas.2007.1s.823.
34 Pawson, M. G., G. D. Pickett, and D. F. Kelley. 1987. The distribution and migrations of bass, Dicentrarchus labrax L., in waters around England and Wales as shown by tagging. Journal of the Marine Biological Association of the United Kingdom 67: 183. https://doi.org/10.1017/S0025315400026448.
35 Varsamos, S., G. Flik, J.F. Pepin, S.E. Wendelaar Bonga, and G. Breuil. 2006. Husbandry stress during early life stages affects the stress response and health status of juvenile sea bass, Dicentrarchus labrax. Fish & Shellfish Immunology 20: 83–96. https://doi.org/10.1016/j.fsi.2005.04.005.
36 Kavadias, S., J. Castritsi‐Catharios, and A. Dessypris. 2003. Annual cycles of growth rate, feeding rate, food conversion, plasma glucose and plasma lipids in a population of European sea bass (Dicentrarchus labrax L.) farmed in floating marine cages. Journal of Applied Ichthyology 19: 29–34. https://doi.org/10.1046/j.1439-0426.2003.00346.x.
37 Hunt, Darcie Elizabeth. 2015. The effect of visual capacity and swimming ability of fish on the performance of light-based bycatch reduction devices in prawn trawls. Doctoral dissertation, University of Tasmania.
38 Pawson, M. G., M. Brown, J. Leballeur, and G. D. Pickett. 2008. Will philopatry in sea bass, Dicentrarchus labrax, facilitate the use of catch-restricted areas for management of recreational fisheries? Fisheries Research 93: 240–243. https://doi.org/10.1016/j.fishres.2008.03.002.
39 Dando, P. R., and Necla Demir. 1985. On the Spawning and Nursery Grounds of Bass, Dicentrarchus Labrax, in the Plymouth Area. Journal of the Marine Biological Association of the United Kingdom 65: 159–168. https://doi.org/10.1017/S0025315400060872.
40 Gorshkov, S., G. Gorshkova, and W. R. Knibb. 1999. Sex ratios and growth performance of European sea bass (Dicentrarchus labrax L.) reared in mariculture in Eilat (Red Sea). Israeli Journal of Aquaculture - Bamidgeh 51: 91–105.
41 Bagni, M., C. Civitareale, A. Priori, A. Ballerini, M. Finoia, G. Brambilla, and G. Marino. 2007. Pre-slaughter crowding stress and killing procedures affecting quality and welfare in sea bass (Dicentrarchus labrax) and sea bream (Sparus aurata). Aquaculture 263: 52–60. https://doi.org/10.1016/j.aquaculture.2006.07.049.
42 Acerete, L., L. Reig, D. Alvarez, R. Flos, and L. Tort. 2009. Comparison of two stunning/slaughtering methods on stress response and quality indicators of European sea bass (Dicentrarchus labrax). Aquaculture 287: 139–144. https://doi.org/10.1016/j.aquaculture.2008.10.012.
43 Di Marco, P., A. Priori, M.G. Finoia, A. Massari, A. Mandich, and G. Marino. 2008. Physiological responses of European sea bass Dicentrarchus labrax to different stocking densities and acute stress challenge. Aquaculture 275: 319–328. https://doi.org/10.1016/j.aquaculture.2007.12.012.
44 Lambooij, Bert, Marien A Gerritzen, Henny Reimert, Dirk Burggraaf, Geert André, and Hans Van De Vis. 2008. Evaluation of electrical stunning of sea bass (Dicentrarchus labrax) in seawater and killing by chilling: welfare aspects, product quality and possibilities for implementation. Aquaculture Research 39: 50–58. https://doi.org/10.1111/j.1365-2109.2007.01860.x.
45 Fatira, E., N. Papandroulakis, and M. Pavlidis. 2013. Diel changes in plasma cortisol and effects of size and stress duration on the cortisol response in European sea bass (Dicentrarchus labrax). Fish Physiology and Biochemistry 40: 911–919. https://doi.org/10.1007/s10695-013-9896-1.
46 Person-Le Ruyet, Jeannine, and Nicolas Le Bayon. 2009. Effects of temperature, stocking density and farming conditions on fin damage in European sea bass (Dicentrarchus labrax). Aquatic Living Resources 22: 349–362. https://doi.org/10.1051/alr/2009047.
47 Lovell, J. M., M. M. Findlay, G. Harper, R. M. Moate, and D. A. Pilgrim. 2005. The polarisation of hair cells from the ear of the European bass (Dicentrarchus labrax). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 141: 116–121. https://doi.org/10.1016/j.cbpb.2005.04.010.
48 Brown, Culum. 2015. Fish intelligence, sentience and ethics. Animal Cognition 18: 1–17. https://doi.org/10.1007/s10071-014-0761-0.
49 Amundsen, Lasse, and Martin Landro. 2011. Marine seismic sources part VIII: Fish hear a great deal. Recent Advances in Technology 8: 1–5.
50 Sammouth, Sophie, Emmanuelle Roque d’Orbcastel, Eric Gasset, Gilles Lemarié, Gilles Breuil, Giovanna Marino, Jean-Luc Coeurdacier, Sveinung Fivelstad, and Jean-Paul Blancheton. 2009. The effect of density on sea bass (Dicentrarchus labrax) performance in a tank-based recirculating system. Aquacultural Engineering 40: 72–78. https://doi.org/10.1016/j.aquaeng.2008.11.004.
51 Réale, Denis, Simon M. Reader, Daniel Sol, Peter T. McDougall, and Niels J. Dingemanse. 2007. Integrating animal temperament within ecology and evolution. Biological Reviews 82: 291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x.
52 Vazzana, M, M Cammarata, E.L Cooper, and N Parrinello. 2002. Confinement stress in sea bass (Dicentrarchus labrax) depresses peritoneal leukocyte cytotoxicity. Aquaculture 210: 231–243. https://doi.org/10.1016/S0044-8486(01)00818-3.
53 Maricchiolo, Giulia, Simone Mirto, Gabriella Caruso, Tiziana Caruso, Rosa Bonaventura, Monica Celi, Valeria Matranga, and Lucrezia Genovese. 2011. Welfare status of cage farmed European sea bass (Dicentrarchus labrax): A comparison between submerged and surface cages. Aquaculture 314: 173–181. https://doi.org/10.1016/j.aquaculture.2011.02.001.
54 Robb, D H F, and S C Kestin. 2002. Methods Used to Kill Fish: Field Observations and Literature Reviewed. Animal Welfare 11: 269–282.
55 Simitzis, Panagiotis E, Aristeidis Tsopelakos, Maria A Charismiadou, Alkisti Batzina, Stelios G Deligeorgis, and Helen Miliou. 2013. Comparison of the effects of six stunning/killing procedures on flesh quality of sea bass (Dicentrarchus labrax, Linnaeus 1758) and evaluation of clove oil anaesthesia followed by chilling on ice/water slurry for potential implementation in aquaculture. Aquaculture Research: n/a-n/a. https://doi.org/10.1111/are.12120.









Probably, we updated the profile. Check the version number in the head of the page. For more information on the version, see the FAQ about this. Why do we update profiles? Not just do we want to include new research that has come out, but we are continuously developing the database itself. For example, we changed the structure of entries in criteria or we added explanations for scores in the WelfareCheck | farm. And we are always refining our scoring rules.
The centre of the Overview is an array of criteria covering basic features and behaviours of the species. Each of this information comes from our literature search on the species. If we researched a full Dossier on the species, probably all criteria in the Overview will be covered and thus filled. This was our way to go when we first set up the database.
Because Dossiers are time consuming to research, we switched to focusing on WelfareChecks. These are much shorter profiles covering just 10 criteria we deemed important when it comes to behaviour and welfare in aquaculture (and lately fisheries, too). Also, WelfareChecks contain the assessment of the welfare potential of a species which has become the main feature of the fair-fish database over time. Because WelfareChecks do not cover as many criteria as a Dossier, we don't have the information to fill all blanks in the Overview, as this information is "not investigated by us yet".
Our long-term goal is to go back to researching Dossiers for all species covered in the fair-fish database once we set up WelfareChecks for each of them. If you would like to support us financially with this, please get in touch at ffdb@fair-fish.net
See the question "What does "not investigated by us yet" mean?". In short, if we have not had a look in the literature - or in other words, if we have not investigated a criterion - we cannot know the data. If we have already checked the literature on a criterion and could not find anything, it is "no data found yet". You spotted a "no data found yet" where you know data exists? Get in touch with us at ffdb@fair-fish.net!
Once you have clicked on "show details", the entry for a criterion will unfold and display the summarised information we collected from the scientific literature – complete with the reference(s).
As reference style we chose "Springer Humanities (numeric, brackets)" which presents itself in the database as a number in a grey box. Mouse over the box to see the reference; click on it to jump to the bibliography at the bottom of the page. But what does "[x]-[y]" refer to?
This is the way we mark secondary citations. In this case, we read reference "y", but not reference "x", and cite "x" as mentioned in "y". We try to avoid citing secondary references as best as possible and instead read the original source ourselves. Sometimes we have to resort to citing secondarily, though, when the original source is: a) very old or not (digitally) available for other reasons, b) in a language no one in the team understands. Seldomly, it also happens that we are running out of time on a profile and cannot afford to read the original. As mentioned, though, we try to avoid it, as citing mistakes may always happen (and we don't want to copy the mistake) and as misunderstandings may occur by interpreting the secondarily cited information incorrectly.
If you spot a secondary reference and would like to send us the original work, please contact us at ffdb@fair-fish.net
In general, we aim at giving a good representation of the literature published on the respective species and read as much as we can. We do have a time budget on each profile, though. This is around 80-100 hours for a WelfareCheck and around 300 hours for a Dossier. It might thus be that we simply did not come around to reading the paper.
It is also possible, though, that we did have to make a decision between several papers on the same topic. If there are too many papers on one issue than we manage to read in time, we have to select a sample. On certain topics that currently attract a lot of attention, it might be beneficial to opt for the more recent papers; on other topics, especially in basic research on behaviour in the wild, the older papers might be the go-to source.
And speaking of time: the paper you are missing from the profile might have come out after the profile was published. For the publication date, please check the head of the profile at "cite this profile". We currently update profiles every 6-7 years.
If your paper slipped through the cracks and you would like us to consider it, please get in touch at ffdb@fair-fish.net
This number, for example "C | 2.1 (2022-11-02)", contains 4 parts:
- "C" marks the appearance – the design level – of the profile part. In WelfareChecks | farm, appearance "C" is our most recent one with consistent age class and label (WILD, FARM, LAB) structure across all criteria.
- "2." marks the number of major releases within this appearance. Here, it is major release 2. Major releases include e.g. changes of the WelfareScore. Even if we just add one paper – if it changes the score for one or several criteria, we will mark this as a major update for the profile. With a change to a new appearance, the major release will be re-set to 1.
- ".1" marks the number of minor updates within this appearance. Here, it is minor update 1. With minor updates, we mean changes in formatting, grammar, orthography. It can also mean adding new papers, but if these papers only confirm the score and don't change it, it will be "minor" in our book. With a change to a new appearance, the minor update will be re-set to 0.
- "(2022-11-02)" is the date of the last change – be it the initial release of the part, a minor, or a major update. The nature of the changes you may find out in the changelog next to the version number.
If an Advice, for example, has an initial release date and then just a minor update date due to link corrections, it means that – apart from correcting links – the Advice has not been updated in a major way since its initial release. Please take this into account when consulting any part of the database.
First up, you will find answers to questions for the specific page you are on. Scrolling down in the FAQ window, there are also answers to more general questions. Explore our website and the other sub pages and find there the answers to questions relevant for those pages.
In the fair-fish database, when you have chosen a species (either by searching in the search bar or in the species tree), the landing page is an Overview, introducing the most important information to know about the species that we have come across during our literatures search, including common names, images, distribution, habitat and growth characteristics, swimming aspects, reproduction, social behaviour but also handling details. To dive deeper, visit the Dossier where we collect all available ethological findings (and more) on the most important aspects during the life course, both biologically and concerning the habitat. In contrast to the Overview, we present the findings in more detail citing the scientific references.
Depending on whether the species is farmed or wild caught, you will be interested in different branches of the database.
Farm branch
Founded in 2013, the farm branch of the fair-fish database focuses on farmed aquatic species.
Catch branch
Founded in 2022, the catch branch of the fair-fish database focuses on wild-caught aquatic species.
The heart of the farm branch of the fair-fish database is the welfare assessment – or WelfareCheck | farm – resulting in the WelfareScore | farm for each species. The WelfareCheck | farm is a condensed assessment of the species' likelihood and potential for good welfare in aquaculture, based on welfare-related findings for 10 crucial criteria (home range, depth range, migration, reproduction, aggregation, aggression, substrate, stress, malformations, slaughter).
For those species with a Dossier, we conclude to-be-preferred farming conditions in the Advice | farm. They are not meant to be as detailed as a rearing manual but instead, challenge current farming standards and often take the form of what not to do.
In parallel to farm, the main element of the catch branch of the fair-fish database is the welfare assessment – or WelfareCheck | catch – with the WelfareScore | catch for each species caught with a specific catching method. The WelfareCheck | catch, too, is a condensed assessment of the species' likelihood and potential for good welfare – or better yet avoidance of decrease of good welfare – this time in fisheries. We base this on findings on welfare hazards in 10 steps along the catching process (prospection, setting, catching, emersion, release from gear, bycatch avoidance, sorting, discarding, storing, slaughter).
In contrast to the farm profiles, in the catch branch we assess the welfare separately for each method that the focus species is caught with. In the case of a species exclusively caught with one method, there will be one WelfareCheck, whereas in other species, there will be as many WelfareChecks as there are methods to catch the species with.
Summarising our findings of all WelfareChecks | catch for one species in Advice | catch, we conclude which catching method is the least welfare threatening for this species and which changes to the gear or the catching process will potentially result in improvements of welfare.
Welfare of aquatic species is at the heart of the fair-fish database. In our definition of welfare, we follow Broom (1986): “The welfare of an individual is its state as regards its attempts to cope with its environment.” Thus, welfare may be perceived as a continuum on which an individual rates “good” or “poor” or everything in between.
We pursue what could be called a combination of not only a) valuing the freedom from injuries and stress (function-based approach) but b) supporting attempts to provide rewarding experiences and cognitive challenges (feelings-based approach) as well as c) arguing for enclosures that mimic the wild habitat as best as possible and allow for natural behaviour (nature-based approach).
Try mousing over the element you are interested in - oftentimes you will find explanations this way. If not, there will be FAQ on many of the sub-pages with answers to questions that apply to the respective sub-page. If your question is not among those, contact us at ffdb@fair-fish.net.
It's right here! We decided to re-name it to fair-fish database for several reasons. The database has grown beyond dealing purely with ethology, more towards welfare in general – and so much more. Also, the partners fair-fish and FishEthoGroup decided to re-organise their partnership. While maintaining our friendship, we also desire for greater independence. So, the name "fair-fish database" establishes it as a fair-fish endeavour.