Information
Author: Jenny Volstorf
Version: B | 1.1Published: 2022-01-22
1 Remarks
1.1 General remarks
Escapees and consequences: negative or at most unpredictable for the local ecosystem1.2 Other remarks
No data found yet.2 Ethograms
In the farm or lab: on feeding, daily rhythm, swimming, social behaviour, cognitive abilities, coping styles, stress reactions
3 Distribution
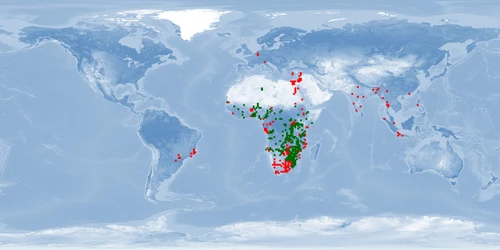
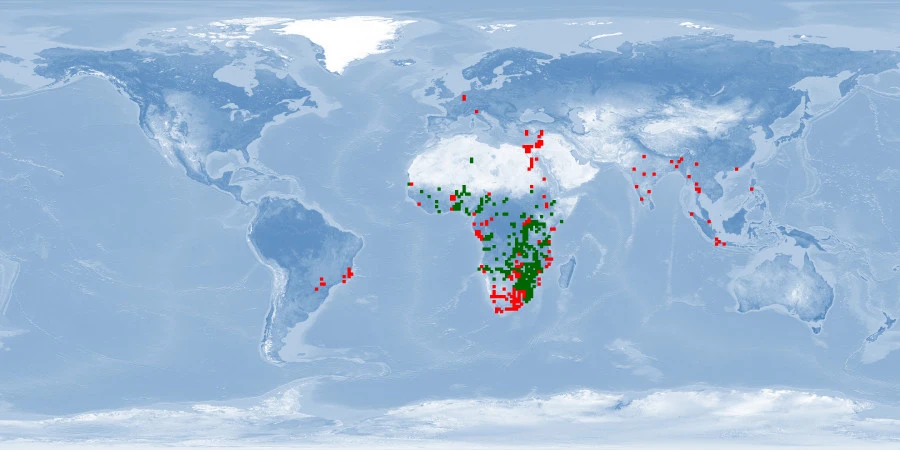
Natural distribution: Africa, Israel
Introduced: inland waters Africa, Asia, South America
4 Natural co-existence
5 Substrate and/or shelter
5.1 Substrate
Substrate range, substrate preference: opportunistic – reported from areas with plants as well as dams with mud and shale bottom, lakes with sandy bottomSubstrate and growth: direct effect, but depends on kind of substrate (further research needed)
5.2 Shelter or cover
Shelter or cover preference: vegetation, occasionally rocks and mud banks (further research needed)Shelter or cover and stress: inverse effect (further research needed)
Shelter or cover and growth: direct effect
6 Food, foraging, hunting, feeding
6.1 Trophic level and general considerations on food needs
Trophic level: 3.8Impacts of feed fishery: contributes to overfishing, challenges animal welfare
6.2 Food items
Food items, food preference: opportunistic – carnivorous, omnivorous if need be; increasing prey size with increasing age6.3 Feeding behaviour
Feeding style, foraging mode: depending on diet either ram feeding or active pursuitFeeding frequency and stress: direct relation (further research needed)
Feeding frequency and growth: benefits from continuous feeding in sync with daily rhythm (further research needed)
Feed delivery and growth: spatially dispersed delivery benefits growth (further research needed)
Food competition and stress: direct effect (further research needed)
Effects on feeding: direct relation with temperature
For feeding and...
...taste → D7,
...social structure → D11,
...shyness-boldness continuum → D12,
...exploration-avoidance continuum → D13,
...handling → D14.
7 Photoperiod
7.1 Daily rhythm
Daily rhythm: mainly nocturnal, differences between individuals and seasonsPhotoperiod and stress: direct relation, depending on light intensity
Photoperiod and growth: inverse relation
7.2 Light intensity
Light intensity preference: 0 lux (further research needed)Light intensity and stress: direct relation (further research needed)
7.3 Light colour
Light colour and stress: blue light is advantageous (further research needed)8 Water parameters
8.1 Water temperature
Standard temperature range, temperature preference: 10-41 °C, unclear preferenceTemperature and stress: decreasing survival <23 °C, but depends on age and way of temperature change (further research needed)
8.2 Oxygen
Dissolved oxygen range: 61-82% saturation, 5.1-6.9 mg/L8.3 Salinity
Salinity tolerance, standard salinity range: 1.0-1.2 mg/L (further research needed)8.4 pH
Standard pH range: 7-9.28.5 Turbidity
Standard turbidity range: 18.9-257 ntu, 533.3-1,256 ppm total dissolved solids, Secchi depth 0.05-3.5 m (further research needed)8.6 Water hardness
No data found yet.8.7 NO4
No data found yet.8.8 Other
No data found yet.9 Swimming
9.1 Swimming type, swimming mode
Swimming type, swimming mode: anguilliform9.2 Swimming speed
Swimming speed: 105-478 m/h, 1.5-9.5 total length/s (fry) (further research needed)Standard velocity range, velocity preference: linear relationship between size and maximum current velocity (further research needed)
9.3 Home range
Home range: 2-70 ha9.4 Depth
Depth range, depth preference: juveniles 0.1-10 m, adults until 40 m, moves shallower during the night9.5 Migration
Migration type: potamodromous10 Growth
10.1 Ontogenetic development
Mature egg: 24-48 h from fertilisation until hatching, 0.1-1.9 mm (further research needed)Larvae: hatching to 2.5-3 days, 3.6-8.5 mm, 1.5+ mg
Fry: beginning of exogenous feeding, 2-70 days, 6.2-42.9 mm, 0.003-0.7 g
Juveniles, sexual maturity: fully developed (25-75 days) to beginning of maturity (0-6 years), 4-92 cm, 1-750 g
Adults: 0-25 years, 35-124 cm, 0.3-8.8 kg
10.2 Sexual conversion
No data found yet.10.3 Sex ratio
Natural male:female ratio: 1:1-1:1.5; 1:1-3.8:110.4 Effects on growth
Growth rate: 20-30+ cm/year in first year, then large variationGrowth and sex: bimodal pattern, noticeable from 0-3 years on
Growth and size-grading: no effect (further research needed)
Growth and other factors: inverse effect of polyculture, but benefit for Oreochromis niloticus; direct effect of larger diameter-to-depth-ratio (further research needed)
For growth and...
...substrate → D22,
...cover → D23,
...feeding frequency → D17,
...feed delivery → D24,
...PHOTOPERIOD → D25,
...tank colour → D3,
...stocking density → D26,
...coping styles → D12.
10.5 Deformities and malformations
Deformities and malformations: head and body abnormalities in 0-100% of individuals (further research needed)Hypotheses regarding causes for deformities: cryopreservation of sperm causing haploidy, stress (further research needed)
11 Reproduction
11.1 Nest building
Nest building: none11.2 Attraction, courtship, mating
Courtship sequence: male slightly butts female who leads him to spawning site, both butt each other (further research needed)11.3 Spawning
Spawning conditions: submerged vegetation, summer, mainly at night, usually >22 °C, fresh water, flooded grassland until <400 mm pools, density 30-60 ind/125 m shoreline (further research needed)Spawning sequence: male and female remain in quasi amplexus, release eggs and sperm in water (further research needed)
Effects on spawning: direct effect of low density, 25 cm water depth (further research needed)
11.4 Fecundity
Female fecundity: range 2,000-650,000 eggs/female, 339,000-1,176,000 eggs/kgEffects on fecundity: inverse effect of stress (further research needed)
Fecundity and manipulation: two consecutive injections with Carp pituitary increases fecundity in males but does not induce natural spawning or improve stripping (further research needed)
11.5 Brood care, breeding
Breeding type: lake spawner12 Senses
12.1 Vision
Visible spectrum: blue, red, green; colour vision limited in fry (further research needed)Importance of vision: swimming, "walking" across land, probably less important for foraging (further research needed)
Tank colour and stress: higher survival in black tanks (further research needed)
Tank colour and growth: higher in black tanks with shade (further research needed)
12.2 Olfaction (and taste, if present)
Olfactory spectrum (and gustatory, if present): sour, salty, bitter, amino acids (further research needed)Importance of olfaction: ovarian and gonadosomatic growth, risk perception
12.3 Hearing
No data found yet.12.4 Touch, mechanical sensing
Importance of touch: ovarian growth, foraging (further research needed)12.5 Lateral line
Importance of lateral line: unclear (further research needed)12.6 Electrical sensing
Electrical sensing spectrum: 13 µV/cm to 2.1 mV/cm (further research needed)Importance of electrical sensing: detecting prey and conspecifics, ovarian growth (further research needed)
12.7 Nociception, pain sensing
No data found yet.12.8 Other
No data found yet.13 Communication
13.1 Visual
No data found yet.13.2 Chemical
Signalling stress: alarm cue must exceed certain concentration (further research needed)13.3 Acoustic
No data found yet.13.4 Mechanical
No data found yet.13.5 Electrical
Signal production: one-phase waves for 5-200+ ms (further research needed)Signalling stress: only in encounters with conspecifics, not alone (further research needed)
13.6 Other
No data found yet.14 Social behaviour
14.1 Spatial organisation
Aggregation type: aggregations, seldomly solitary (further research needed)Stocking density in the wild: 1.3-14.4 catch per unit effort, range 0-20 ind/125 m shoreline (further research needed)
Stocking density and stress: mixed effects (further research needed)
Stocking density and growth: mixed effects (further research needed)
14.2 Social organisation
Social organisation type: linear hierarchy (when in small groups)Features of dominance: occupy best feeding sites, larger and more aggressive than subordinates
Features of subordination: try to escape or hardly move, avoid contact with the dominant (further research needed)
14.3 Exploitation
Cannibalism, predation: prevalent, most likely due to beginning of air breathing and change in feed (further research needed)14.4 Facilitation
Cooperation, mutualism: pack hunting14.5 Aggression
Aggression and stocking density: no effect (further research needed)Aggression and stress: may erect pectoral ray when disturbed (further research needed)
Aggression and size-grading: attacks, chases, biting regardless of size-grading (further research needed)
For aggression and...
...feeding frequency → D39,
...PHOTOPERIOD → D38,
...light intensity → D40,
...pre-courtship behaviour → D30,
...electrical communication → D33,
...dominance → D11.
14.6 Territoriality
For territoriality and...
...shelter → D10,
...PHOTOPERIOD → D38,
...light intensity → D41,
...stocking density → D2,
...difference to cannibalism → D5.
15 Cognitive abilities
15.1 Learning
Operant or instrumental conditioning: may be used for managing self-feeder15.2 Memory
No data found yet.15.3 Problem solving, creativity, planning, intelligence
No data found yet.15.4 Other
No data found yet.16 Personality, coping styles
Exploration-avoidance continuum: relationship with feed consumption (further research needed)
Aggressiveness continuum: in establishing hierarchy, dominance-subordination, given stocking density and size-grading
17 Emotion-like states
17.1 Joy
No data found yet.17.2 Relaxation
No data found yet.17.3 Sadness
No data found yet.17.4 Fear
Fear: decrease in feeding (further research needed)18 Self-concept, self-recognition
19 Reactions to husbandry
19.1 Stereotypical and vacuum activities
Stereotypical and vacuum activities: independent of density (further research needed)19.2 Acute stress
Handling: air exposure, collection and weighing is stressful (further research needed)Live transport: stressful (further research needed)
For acute stress and...
...light colour → D46,
...fecundity → D27,
...electrical signalling → D33.
19.3 Chronic stress
Effects on welfare: size-grading affects swimming, resting, feeding behaviour, and aggression (further research needed)For chronic stress and...
...shelter → D10,
...feeding frequency → D39,
...food competition → D37,
...PHOTOPERIOD → D38,
...light intensity → D40,
...water temperature → D47,
...fecundity → D27,
...tank colour → D48,
...stocking density → D2.
19.4 Stunning reactions
Stunning rules: fast, effective, safeStunning methods: electrical stunning (if head only then combined with decapitation) and shooting most effective (further research needed)
Glossary
AGGRESSIVENESS = agonistic reactions towards conspecifics. Tests: mirror image, social interaction/diadic encounters 85.
EXPLORATION-AVOIDANCE = reaction to new situations, e.g. new habitat, new food, novel objects. Referred to as neophobia/neophilia elsewhere. Tests: open field, trappability for first time, novel environment, hole board (time spent with head in holes), novel object 85.
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FINGERLINGS = early juveniles with fully developed scales and working fins, the size of a human finger
FOOD CONVERSION RATIO = (food offered / weight gained)
FRY = larvae from external feeding on
IND = individuals
JUVENILES = fully developed but immature individuals
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening
MILLIARD = 1,000,000,000 56 57
PHOTOPERIOD = duration of daylight
SHYNESS-BOLDNESS = reaction to risky (but not new!) situations, e.g. predators or humans. Referred to as docility, tameness, fearfulness elsewhere. Tests: predator presentation, predator stimulus, threat, trappability (latency to enter a trap for first time can be exploration), resistance to handlers (Trapezov stick test), tonic immobility (catatonic-like death-feigning anti predator response) 85.
TOTAL LENGTH = from snout to tip of caudal fin as compared to fork length (which measures from snout to fork of caudal fin) or standard length (from head to base of tail fin) or body length (from the base of the eye notch to the posterior end of the telson) 59
WILD = setting in the wild
Bibliography
2 Wartenberg, Reece, Olaf L. F. Weyl, Anthony J. Booth, and Henning Winker. 2013. Life-history characteristics of an age-validated established invasive African sharptooth catfish, Clarias gariepinus, population in a warm–temperate African impoundment. African Zoology 48: 318–325. https://doi.org/10.1080/15627020.2013.11407598.
3 Vitule, J. R. S., S. C. Umbria, and J. M. R. Aranha. 2006. Introduction of the African Catfish Clarias gariepinus (BURCHELL, 1822) into Southern Brazil. Biological Invasions 8: 677. https://doi.org/10.1007/s10530-005-2535-8.
4 Kadye, Wilbert Takawira. 2011. Assessing the impacts of invasive non-native African catfish Clarias gariepinus. Doctor of Philosophy, South Africa: Rhodes University.
5 Radhakrishnan, K. V., Zhao Jun Lan, Jun Zhao, Ning Qing, and Xio Lin Huang. 2011. Invasion of the African sharp-tooth catfish Clarias gariepinus (Burchell, 1822) in South China. Biological Invasions 13: 1723–1727. https://doi.org/10.1007/s10530-011-0004-0.
6 Rabelo, Leandro Bonesi, and Lucy Satiko Hashimoto Soares. 2014. Feeding interaction of the non-native African catfish (Clarias gariepinus Burchell, 1822) in Itanhém river estuary, Bahia, Brazil. Brazilian Journal of Oceanography 62: 179–186. https://doi.org/10.1590/S1679-87592014051406203.
7 Hocutt, Charles H. 1989. Seasonal and diel behaviour of radio-tagged Clarias gariepinus in Lake Ngezi, Zimbabwe (Pisces: Clariidae). Journal of Zoology 219: 181–199. https://doi.org/10.1111/j.1469-7998.1989.tb02575.x.
8 Dadebo, Elias. 2000. Reproductive biology and feeding habits of the catfish Clarias gariepinus (Burchell)(Pisces: Clariidae) in Lake Awassa, Ethiopia. SINET: Ethiopian Journal of Science 23: 231–246.
9 Bruton, M. N. 1978. The Habitats and Habitat Preferences of Clarias Gariepinus (pisces: Clariidae) in a Clear Coastal Lake (lake Sibaya, South Africa). Journal of the Limnological Society of Southern Africa 4: 81–88. https://doi.org/10.1080/03779688.1978.9633156.
10 Willoughby, N. G., and D. Tweddle. 1978. The ecology of the catfish Clarias gariepinus and Clarias ngamensis in the Shire Valley, Malawi. Journal of Zoology 186: 507–534. https://doi.org/10.1111/j.1469-7998.1978.tb03936.x.
11 Bruton, M. N. 1979. The breeding biology and early development of Clarias gariepinus (Pisces: Clariidae) in Lake Sibaya, South Africa, with a review of breeding in species of the subgenus Clarias (Clarias). The Transactions of the Zoological Society of London 35: 1–45. https://doi.org/10.1111/j.1096-3642.1979.tb00056.x.
12 Ikpi, Gabriel U., Adetola Jenyo-Oni, and Benedict O. Offem. 2012. Effect of Season on Catch rate, Diet and Aspects of Reproduction of Clarias gariepinus (Teleostei: Clariidae) in a Tropical Waterfalls. Advances in Life Sciences 2: 68–74.
13 Baron, V. D., K. S. Morshnev, V. M. Olshansky, A. A. Orlov, D. S. Pavlov, and I. Teferi. 2001. Observations of the Electric Activity of Silurid Catfishes (Siluriformes) in Lake Chamo (Ethiopia). Journal of Ichthyology 41: 536–543.
14 NOT FOUND
15 Adámek, Z., K. Fasaic, and M. A. Siddiqui. 1999. Prey selectivity in wels (Silurus glanis) and African catfish (Clarias gariepinus). Ribarstvo 57: 47–60.
16 Kasumyan, A. O. 2014. Behavior and gustatory reception of air-breathing catfishes (Clariidae). Journal of Ichthyology 54: 934–943. https://doi.org/10.1134/S0032945214100075.
17 Britz, P. J., and A. G. Pienaar. 1992. Laboratory experiments on the effect of light and cover on the behaviour and growth of African catfish, Clarias gariepinus (Pisces: Clariidae). Journal of Zoology 227: 43–62. https://doi.org/10.1111/j.1469-7998.1992.tb04343.x.
18 Almazán-Rueda, P., A. T. M Van Helmond, J. a. J. Verreth, and J. W. Schrama. 2005. Photoperiod affects growth, behaviour and stress variables in Clarias gariepinus. Journal of Fish Biology 67: 1029–1039. https://doi.org/10.1111/j.0022-1112.2005.00806.x.
19 Fatollahi, M., and A. O. Kasumyan. 2006. The study of sensory bases of the feeding behavior of the African catfish Clarias gariepinus (Clariidae, Siluriformes). Journal of Ichthyology 46: S161–S172. https://doi.org/10.1134/S0032945206110051.
20 Mauguit, Quentin, Vincent Gennotte, Christophe Becco, Etienne Baras, Nicolas Vandewalle, and Pierre Vandewalle. 2010. Ontogeny of swimming movements in the catfish Clarias gariepinus. Open Fish Science Journal 3. https://doi.org/10.2174/1874401X01003010016.
21 Baron, V. D., A. A. Orlov, and A. S. Golubtsov. 1994. African Clarias catfish elicits long-lasting weak electric pulses. Experientia 50: 644–647. https://doi.org/10.1007/BF01952864.
22 Lee, C. K., G. Kawamura, S. Senoo, F. F. Ching, and M. Luin. 2014. Colour vision in juvenile African catfish Clarias gariepinus. International Research Journal of Biological Sciences 3: 36–41.
23 Haylor, G. S. 1992. Controlled hatchery production of Clarias gariepinus (Burchell): growth and survival of larvae at high stocking density. Aquaculture Research 23: 303–314. https://doi.org/10.1111/j.1365-2109.1992.tb00773.x.
24 Haylor, Graham S. 1992. The culture of African Catfish, Clarias gariepinus (Burchell) in Africa, with particular reference to controlled hatchery production. Doctoral dissertation, Stirling, Scotland: University of Stirling.
25 Solomon, S. G., and V. T. Okomoda. 2012. Growth response and aggressive behaviour of Clarias gariepinus fingerlings reared at different photoperiods in a water recirculatory system. Livestock Research for Rural Development 24.
26 Kaiser, H., O. Weyl, and T. Hecht. 1995. Observations on agonistic behaviour of Clarias gariepinus larvae and juveniles under different densities and feeding frequencies in a controlled environment. Journal of Applied Ichthyology 11: 25–36. https://doi.org/10.1111/j.1439-0426.1995.tb00003.x.
27 Almazán-Rueda, Pablo, Johan W Schrama, and Johan A. J Verreth. 2004. Behavioural responses under different feeding methods and light regimes of the African catfish (Clarias gariepinus) juveniles. Aquaculture 231: 347–359. https://doi.org/10.1016/j.aquaculture.2003.11.016.
28 van de Nieuwegiessen, Pascal G., Johan W. Schrama, and Johan A. J. Verreth. 2008. A note on alarm cues in juvenile African catfish, Clarias gariepinus Burchell: Indications for opposing behavioural strategies. Applied Animal Behaviour Science 113: 270–275. https://doi.org/10.1016/j.applanim.2007.11.008.
29 van de Nieuwegiessen, P. G., N. M. Ramli, B. P. F. J. M. Knegtel, J. A. J. Verreth, and J. W. Schrama. 2010. Coping strategies in farmed African catfish Clarias gariepinus. Does it affect their welfare? Journal of Fish Biology 76: 2486–2501. https://doi.org/10.1111/j.1095-8649.2010.02635.x.
30 Martins, Catarina I. M., Margaret Aanyu, Johan W. Schrama, and Johan A. J. Verreth. 2005. Size distribution in African catfish (Clarias gariepinus) affects feeding behaviour but not growth. Aquaculture 250: 300–307. https://doi.org/10.1016/j.aquaculture.2005.05.034.
31 Adebayo, OT. 2006. Reproductive performance of African Clariid Catfish Clarias gariepinus broodstock on varying maternal stress. Journal of Fisheries international 1: 17–20.
32 Martins, Catarina I. M., Johan W. Schrama, and Johan A. J. Verreth. 2006. The effect of group composition on the welfare of African catfish (Clarias gariepinus). Applied Animal Behaviour Science 97: 323–334. https://doi.org/10.1016/j.applanim.2005.07.003.
33 van de Nieuwegiessen, Pascal G., Annette S. Boerlage, Johan A. J. Verreth, and Johan W. Schrama. 2008. Assessing the effects of a chronic stressor, stocking density, on welfare indicators of juvenile African catfish, Clarias gariepinus Burchell. Applied Animal Behaviour Science 115: 233–243. https://doi.org/10.1016/j.applanim.2008.05.008.
34 van de Nieuwegiessen, Pascal G., Jacob Olwo, Sophoan Khong, Johan A. J. Verreth, and Johan W. Schrama. 2009. Effects of age and stocking density on the welfare of African catfish, Clarias gariepinus Burchell. Aquaculture 288: 69–75. https://doi.org/10.1016/j.aquaculture.2008.11.009.
35 Sallehudin, Firdaus, and Yukinori Mukai. 2014. Cannibalistic behaviour of African catfish juveniles, Clarias gariepinus under different light wavelengths and intensities. In Proceeding of the 3rd International Conference on Applied Life Sciences, 51–55. Malaysia: ISALS Publishing.
36 Shourbela, Ramy M., Ashraf M. Abd El-latif, and Eman A. Abd El-Gawad. 2016. Are Pre Spawning Stressors Affect Reproductive Performance of African Catfish Clarias gariepinus? Turkish Journal of Fisheries and Aquatic Sciences 16: 651–657.
37 Reviewed distribution maps for African catfish (Clarias gariepinus). 2016. Aquamaps.
38 Dadebo, E. 2009. Filter-feeding habit of the African catfish Clarias gariepinus (Burchell, 1822)(Pisces: Clariidae) in Lake Chamo, Ethiopia. Ethiop. J. Biol. Sci. 8: 15–30.
39 Mbalassa, Mulongaibalu, Muderhwa Nshombo, Mujugu Eliezer Kateyo, Lauren Chapman, Jackson Efitre, and Gladys Bwanika. 2015. Identification of migratory and spawning habitats of Clarias gariepinus (Burchell, 1822) in Lake Edward-Ishasha River watershed, Albertine Rift Valley, East Africa. International Journal of Fisheries and Aquatic Studies 2: 128–138.
40 Bruton, M. N., and B. R. Allanson. 1980. Growth of Clarias gariepinus in Lake Sibaya, South Africa. African Zoology 15: 7–15.
41 Megbowon, I., H. A. Fashina-Bombata, M. M.-A. Akinwale, A. M. Hammed, T. O. Mojekwu, O. A. Okunade, and R. O. D. Shell. 2013. Growth performance of wild strains of Clarias gariepinus from Nigerian waters. In , 65–67. Lagos (Nigeria): FISON.
42 Bokhutlo, Thethela, Olaf L. F. Weyl, Ketlhatlogile Mosepele, and G. Glenn Wilson. 2015. Age and growth of sharptooth catfish, Clarias gariepinus (Burchell, 1822) (Clariidae), in the Lower Okavango Delta, Botswana. Marine and Freshwater Research 66: 420–428. https://doi.org/10.1071/MF13322.
43 Quick, A. J. R., and M. N. Bruton. 1984. Age and growth of Clarias gariepinus (Pisces: Clariidae) in the P.K. le Roux Dam, South Africa. South African Journal of Zoology 19: 37–45. https://doi.org/10.1080/02541858.1984.11447854.
44 Van den Hurk, R., W. J. A. R. Viveen, R. Pinkas, and P. G. W. J. van Oordt. 1985. The Natural Gonadal Cycle in the African Catfish Clarias Gariepinus; a Basis for Applied Studies on Its Reproduction in Fish Farms. Israel Journal of Zoology 33: 129–147. https://doi.org/10.1080/00212210.1985.10688566.
45 Krishnakumar, K., A. Ali, B. Pereira, and R. Raghavan. 2011. Unregulated aquaculture and invasive alien species: a case study of the African Catfish Clarias gariepinus in Vembanad Lake (Ramsar Wetland), Kerala, India. Journal of Threatened Taxa 3: 1737–1744. https://doi.org/10.11609/JoTT.o2378.1737-44.
46 Alves, Carlos Bernardo Mascarenhas, Volney Vono, and Fábio Vieira. 1999. Presence of the walking catfish Clarias gariepinus (Burchell) (Siluriformes, Clariidae) in Minas Gerais state hydrographie basins, Brazil. Revista Brasileira de Zoologia 16: 259–263. https://doi.org/10.1590/S0101-81751999000100022.
47 Rocha, Gecely Rodrigues Alves. 2008. The introduction of the African catfish Clarias gariepinus (Burchell, 1822) into Brazilian inland waters: a growing threat. Neotropical Ichthyology 6: 693–696. https://doi.org/10.1590/S1679-62252008000400020.
48 Anteneh, Wassie, Eshete Dejen, and Abebe Getahun. 2012. Shesher and Welala Floodplain Wetlands (Lake Tana, Ethiopia): Are They Important Breeding Habitats for Clarias gariepinus and the Migratory Labeobarbus Fish Species? The Scientific World Journal. https://doi.org/10.1100/2012/298742.
49 Macharia, S. K., C. C. Ngugi, and J. Rasowo. 2005. Comparative study of hatching rates of African catfish (Clarias gariepinus Burchell 1822) eggs on different substrates. Naga, Worldfish Center Quarterly 28: 23–26.
50 Amisah, S., D. Adjei-Boateng, and D. D. Afianu. 2008. Effects of bamboo substrate and supplementary feed on growth and production of the African catfish, Clarias gariepinus. Journal of Applied Sciences and Environmental Management 12. https://doi.org/10.4314/jasem.v12i2.55521.
51 Froese, R., and D. Pauly. 2014. FishBase. World Wide Web electronic publication. www.fishbase.org.
52 FAO. 2014. The State of World Fisheries and Aquaculture 2014. Rome: Food and Agriculture Organization of the United Nations.
53 Watson, R., Jackie Alder, and Daniel Pauly. 2006. Fisheries for forage fish, 1950 to the present. In On the Multiple Uses of Forage Fish: from Ecosystems to Markets, ed. Jackie Alder and Daniel Pauly, 14:1–20. Fisheries Centre Research Reports 3. Vancouver, Canada: Fisheries Centre, University of British Columbia.
54 Mood, A. 2012. Average annual fish capture for species mostly used for fishmeal (2005-2009). fishcount.org.uk.
55 Mood, A., and P. Brooke. 2012. Estimating the Number of Farmed Fish Killed in Global Aquaculture Each Year.
56 Kopf, Von Kristin. 2012. Milliarden vs. Billionen: Große Zahlen. Sprachlog.
57 Weisstein, Eric W. 2018. Milliard. Text. MathWorld - a Wolfram Web resource. http://mathworld.wolfram.com/Milliard.html. Accessed February 2.
58 Yilmaz, Erdal, Ahmet Bozkurt, and Kaya Gökçek. 2006. Prey Selection by African Catfish Clarias gariepinus (Burchell, 1822) Larvae Fed Different Feeding Regimes. TURKISH JOURNAL OF ZOOLOGY 30: 59–66.
59 Pawson, M.G., and G.D. Pickett. 1996. The Annual Pattern of Condition and Maturity in Bass, Dicentrarchus Labrax, in Waters Around England and Wales. Journal of the Marine Biological Association of the United Kingdom 76: 107. https://doi.org/10.1017/S0025315400029040.
60 Hossain, Mostafa A. R., Graham S. Haylor, and Malcolm C. M. Beveridge. 2001. Effect of feeding time and frequency on the growth and feed utilization of African catfish Clarias gariepinus (Burchell 1822) fingerlings. Aquaculture Research 32: 999–1004. https://doi.org/10.1046/j.1365-2109.2001.00635.x.
61 Rad, Feri̇t, Gülderen Kurt, and A. Sezai̇ Bozaoğlu. 2004. Effects of Spatially Localized and Dispersed Patterns of Feed Distribution on the Growth, Size Dispersion and Feed Conversion Ratio of the African Catfish (Clarias gariepinus). TURKISH JOURNAL OF VETERINARY AND ANIMAL SCIENCES 28: 851–856.
62 Greenwood, P. H. 1992. Personal communication.
63 Britz, P. J., and A. G. Pienaar. 1992. Personal observation.
64 Uys, W. 1992. Personal communication.
65 Hoffman, L. C., J. F. Prinsloo, D. M. Pretorius, and J. Theron. 1991. Observations on the effects of decreasing water temperatures on survival of Clarias gariepinus juveniles. South African Journal of Wildlife Research - 24-month delayed open access 21: 54–58.
66 Adeyemo, O. K., S. A. Agbede, A. O. Olaniyan, and O. A. Shoaga. 2003. The haematological response of Clarias Gariepinus to changes in acclimation temperature. African Journal of Biomedical Research 6. https://doi.org/10.4314/ajbr.v6i2.54033.
67 Miskolczi, Edit, Szilvia Mihálffy, Eszter Patakiné Várkonyi, Béla Urbányi, and Ákos Horváth. 2005. Examination of larval malformations in African catfish Clarias gariepinus following fertilization with cryopreserved sperm. Aquaculture 247. Genetics In Aquaculture VIII: 119–125. https://doi.org/10.1016/j.aquaculture.2005.02.043.
68 Çek, Şehri̇ban, and Erdal Yilmaz. 2007. Gonad Development and Sex Ratio of Sharptooth Catfish (Clarias gariepinus Burchell, 1822) Cultured under Laboratory Conditions. TURKISH JOURNAL OF ZOOLOGY 31: 35–46.
69 Teye, Charles. 2011. A Comparative Study of the Reproductive and Early Life Growth Performance of Three Stocks of the African Catfish, Clarias Gariepinus, (Burchell, 1822) In Ghana. M. Phil Thesis, Legon: University of Ghana.
70 Adewumi, A. A. 2015. Effect of Shade and Enclosure Colour on Behaviour, Growth and Survival of Clarias gariepinus Fry. Applied Science Report 11: 49–51. https://doi.org/10.15192/PSCP.ASR.2015.11.2.4951.
71 Van Weerd, J. H., M. Sukkel, A. B. J. Bongers, H. M. Van Der Does, E. Steynis, and C. J. J. Richter. 1991. Stimulation of gonadal development by sexual interaction of pubertal African catfish, Clarias gariepinus. Physiology & Behavior 49: 217–223. https://doi.org/10.1016/0031-9384(91)90035-M.
72 Van Weerd, J. H., M. Sukkel, and C. J. J. Richter. 1988. An analysis of sex stimuli enhancing ovarian growth in pubertal African catfish, Clarias gariepinus. Aquaculture 75: 181–191. https://doi.org/10.1016/0044-8486(88)90031-2.
73 Martins, Catarina I. M., Johan W. Schrama, and Johan A. J. Verreth. 2006. The relationship between individual differences in feed efficiency and stress response in African catfish Clarias gariepinus. Aquaculture 256: 588–595. https://doi.org/10.1016/j.aquaculture.2006.02.051.
74 Ching, F. F., S. Senoo, and G. Kawamura. 2015. Relative Importance of Vision estimated from the Brain pattern in African catfish Clarias gariepinus, river catfish Pangasius pangasius and red tilapia Oreochromis sp. International Research Journal of Biological Sciences 4: 6–10.
75 Van Weerd, J. H., M. Sukkel, I. Bin Awang Kechik, A. B. J. Bongers, and C. J. J. Richter. 1990. Pheromonal stimulation of ovarian recrudescence in hatchery-raised adult African catfish, Clarias gariepinus. Aquaculture 90: 369–387. https://doi.org/10.1016/0044-8486(90)90260-T.
76 Viveiros, A. T. M, Y Fessehaye, M ter Veld, R. W Schulz, and J Komen. 2002. Hand-stripping of semen and semen quality after maturational hormone treatments, in African catfish Clarias gariepinus. Aquaculture 213: 373–386. https://doi.org/10.1016/S0044-8486(02)00036-4.
77 Shoko, Amon Paul, Samwel Mchele Limbu, Hillary Deogratias John Mrosso, Adolf Faustine Mkenda, and Yunus Daud Mgaya. 2016. Effect of stocking density on growth, production and economic benefits of mixed sex Nile tilapia (Oreochromis niloticus) and African sharptooth catfish (Clarias gariepinus) in polyculture and monoculture. Aquaculture Research 47: 36–50. https://doi.org/10.1111/are.12463.
78 Adewolu, Morenike A., Adetola O. Ogunsanmi, and Abubaka Yunusa. 2008. Studies on Growth Performance and Feed Utilization of Two Clariid Catfish and their Hybrid Reared Under Different Culture Systems. European Journal of Scientific Research 23: 252–260.
79 Alarape, Selim Adewale, Temilolu Oladipo Hussein, Eyihuri Veronica Adetunji, and Olanike Kudirat Adeyemo. 2015. Skeletal and Other Morphological Abnormalities in Cultured Nigerian African Catfish (Clarias Gariepinus, Burchell 1822). International Journal of Fisheries and Aquatic Studies 2: 20–25.
80 El Naggar, Gamal O., George John, Mahmoud A. Rezk, Waheed Elwan, and Mohammed Yehia. 2006. Effect of varying density and water level on the spawning response of African catfish Clarias gariepinus: Implications for seed production. Aquaculture 261: 904–907. https://doi.org/10.1016/j.aquaculture.2006.07.043.
81 de Graaf, G J, F Galemoni, and B Banzoussi. 1995. Artificial reproduction and fingerling production of the African catfish, Clarias gariepinus (Burchell 1822), in protected and unprotected ponds. Aquaculture Research 26: 233–242. https://doi.org/10.1111/j.1365-2109.1995.tb00908.x.
82 Hanika, S., and B. Kramer. 2000. Electrosensory prey detection in the African sharptooth catfish, Clarias gariepinus (Clariidae), of a weakly electric mormyrid fish, the bulldog (Marcusenius macrolepidotus). Behavioral Ecology and Sociobiology 48: 218–228. https://doi.org/10.1007/s002650000232.
83 van de Nieuwegiessen, Pascal G., Heling Zhao, Johan A. J. Verreth, and Johan W. Schrama. 2009. Chemical alarm cues in juvenile African catfish, Clarias gariepinus Burchell: A potential stressor in aquaculture? Aquaculture 286: 95–99. https://doi.org/10.1016/j.aquaculture.2008.09.015.
84 Satora, Leszek, Michal Kuciel, and Tomasz Gawlikowski. 2008. Catfish stings and the venom apparatus of the African catfish Clarias gariepinus (Burchell, 1822) and stinging catfish Heteropneustes fossilis (Bloch, 1794). Ann Agri Environ Med 15: 127–130.
85 Réale, Denis, Simon M. Reader, Daniel Sol, Peter T. McDougall, and Niels J. Dingemanse. 2007. Integrating animal temperament within ecology and evolution. Biological Reviews 82: 291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x.
86 Manuel, Remy, Jeroen Boerrigter, Jonathan Roques, Jan van der Heul, Ruud van den Bos, Gert Flik, and Hans van de Vis. 2014. Stress in African catfish (Clarias gariepinus) following overland transportation. Fish Physiology and Biochemistry 40: 33–44. https://doi.org/10.1007/s10695-013-9821-7.
87 Robb, D H F, and S C Kestin. 2002. Methods Used to Kill Fish: Field Observations and Literature Reviewed. Animal Welfare 11: 269–282.
88 Lambooij, E, R J Kloosterboer, C Pieterse, M A Gerritzen, and J W Van de vis. 2003. Stunning of farmed African catfish (Clarias gariepinus) using a captive needle pistol; assessment of welfare aspects. Aquaculture Research 34: 1353–1358. https://doi.org/10.1046/j.1365-2109.2003.00966.x.
89 Lambooij, E., R. J. Kloosterboer, M. A. Gerritzen, and J. W. van de Vis. 2006. Assessment of electrical stunning in fresh water of African Catfish (Clarias gariepinus) and chilling in ice water for loss of consciousness and sensibility. Aquaculture 254: 388–395. https://doi.org/10.1016/j.aquaculture.2005.10.027.





Probably, we updated the profile. Check the version number in the head of the page. For more information on the version, see the FAQ about this. Why do we update profiles? Not just do we want to include new research that has come out, but we are continuously developing the database itself. For example, we changed the structure of entries in criteria or we added explanations for scores in the WelfareCheck | farm. And we are always refining our scoring rules.
The centre of the Overview is an array of criteria covering basic features and behaviours of the species. Each of this information comes from our literature search on the species. If we researched a full Dossier on the species, probably all criteria in the Overview will be covered and thus filled. This was our way to go when we first set up the database.
Because Dossiers are time consuming to research, we switched to focusing on WelfareChecks. These are much shorter profiles covering just 10 criteria we deemed important when it comes to behaviour and welfare in aquaculture (and lately fisheries, too). Also, WelfareChecks contain the assessment of the welfare potential of a species which has become the main feature of the fair-fish database over time. Because WelfareChecks do not cover as many criteria as a Dossier, we don't have the information to fill all blanks in the Overview, as this information is "not investigated by us yet".
Our long-term goal is to go back to researching Dossiers for all species covered in the fair-fish database once we set up WelfareChecks for each of them. If you would like to support us financially with this, please get in touch at ffdb@fair-fish.net
See the question "What does "not investigated by us yet" mean?". In short, if we have not had a look in the literature - or in other words, if we have not investigated a criterion - we cannot know the data. If we have already checked the literature on a criterion and could not find anything, it is "no data found yet". You spotted a "no data found yet" where you know data exists? Get in touch with us at ffdb@fair-fish.net!
Once you have clicked on "show details", the entry for a criterion will unfold and display the summarised information we collected from the scientific literature – complete with the reference(s).
As reference style we chose "Springer Humanities (numeric, brackets)" which presents itself in the database as a number in a grey box. Mouse over the box to see the reference; click on it to jump to the bibliography at the bottom of the page. But what does "[x]-[y]" refer to?
This is the way we mark secondary citations. In this case, we read reference "y", but not reference "x", and cite "x" as mentioned in "y". We try to avoid citing secondary references as best as possible and instead read the original source ourselves. Sometimes we have to resort to citing secondarily, though, when the original source is: a) very old or not (digitally) available for other reasons, b) in a language no one in the team understands. Seldomly, it also happens that we are running out of time on a profile and cannot afford to read the original. As mentioned, though, we try to avoid it, as citing mistakes may always happen (and we don't want to copy the mistake) and as misunderstandings may occur by interpreting the secondarily cited information incorrectly.
If you spot a secondary reference and would like to send us the original work, please contact us at ffdb@fair-fish.net
In general, we aim at giving a good representation of the literature published on the respective species and read as much as we can. We do have a time budget on each profile, though. This is around 80-100 hours for a WelfareCheck and around 300 hours for a Dossier. It might thus be that we simply did not come around to reading the paper.
It is also possible, though, that we did have to make a decision between several papers on the same topic. If there are too many papers on one issue than we manage to read in time, we have to select a sample. On certain topics that currently attract a lot of attention, it might be beneficial to opt for the more recent papers; on other topics, especially in basic research on behaviour in the wild, the older papers might be the go-to source.
And speaking of time: the paper you are missing from the profile might have come out after the profile was published. For the publication date, please check the head of the profile at "cite this profile". We currently update profiles every 6-7 years.
If your paper slipped through the cracks and you would like us to consider it, please get in touch at ffdb@fair-fish.net
This number, for example "C | 2.1 (2022-11-02)", contains 4 parts:
- "C" marks the appearance – the design level – of the profile part. In WelfareChecks | farm, appearance "C" is our most recent one with consistent age class and label (WILD, FARM, LAB) structure across all criteria.
- "2." marks the number of major releases within this appearance. Here, it is major release 2. Major releases include e.g. changes of the WelfareScore. Even if we just add one paper – if it changes the score for one or several criteria, we will mark this as a major update for the profile. With a change to a new appearance, the major release will be re-set to 1.
- ".1" marks the number of minor updates within this appearance. Here, it is minor update 1. With minor updates, we mean changes in formatting, grammar, orthography. It can also mean adding new papers, but if these papers only confirm the score and don't change it, it will be "minor" in our book. With a change to a new appearance, the minor update will be re-set to 0.
- "(2022-11-02)" is the date of the last change – be it the initial release of the part, a minor, or a major update. The nature of the changes you may find out in the changelog next to the version number.
If an Advice, for example, has an initial release date and then just a minor update date due to link corrections, it means that – apart from correcting links – the Advice has not been updated in a major way since its initial release. Please take this into account when consulting any part of the database.
First up, you will find answers to questions for the specific page you are on. Scrolling down in the FAQ window, there are also answers to more general questions. Explore our website and the other sub pages and find there the answers to questions relevant for those pages.
In the fair-fish database, when you have chosen a species (either by searching in the search bar or in the species tree), the landing page is an Overview, introducing the most important information to know about the species that we have come across during our literatures search, including common names, images, distribution, habitat and growth characteristics, swimming aspects, reproduction, social behaviour but also handling details. To dive deeper, visit the Dossier where we collect all available ethological findings (and more) on the most important aspects during the life course, both biologically and concerning the habitat. In contrast to the Overview, we present the findings in more detail citing the scientific references.
Depending on whether the species is farmed or wild caught, you will be interested in different branches of the database.
Farm branch
Founded in 2013, the farm branch of the fair-fish database focuses on farmed aquatic species.
Catch branch
Founded in 2022, the catch branch of the fair-fish database focuses on wild-caught aquatic species.
The heart of the farm branch of the fair-fish database is the welfare assessment – or WelfareCheck | farm – resulting in the WelfareScore | farm for each species. The WelfareCheck | farm is a condensed assessment of the species' likelihood and potential for good welfare in aquaculture, based on welfare-related findings for 10 crucial criteria (home range, depth range, migration, reproduction, aggregation, aggression, substrate, stress, malformations, slaughter).
For those species with a Dossier, we conclude to-be-preferred farming conditions in the Advice | farm. They are not meant to be as detailed as a rearing manual but instead, challenge current farming standards and often take the form of what not to do.
In parallel to farm, the main element of the catch branch of the fair-fish database is the welfare assessment – or WelfareCheck | catch – with the WelfareScore | catch for each species caught with a specific catching method. The WelfareCheck | catch, too, is a condensed assessment of the species' likelihood and potential for good welfare – or better yet avoidance of decrease of good welfare – this time in fisheries. We base this on findings on welfare hazards in 10 steps along the catching process (prospection, setting, catching, emersion, release from gear, bycatch avoidance, sorting, discarding, storing, slaughter).
In contrast to the farm profiles, in the catch branch we assess the welfare separately for each method that the focus species is caught with. In the case of a species exclusively caught with one method, there will be one WelfareCheck, whereas in other species, there will be as many WelfareChecks as there are methods to catch the species with.
Summarising our findings of all WelfareChecks | catch for one species in Advice | catch, we conclude which catching method is the least welfare threatening for this species and which changes to the gear or the catching process will potentially result in improvements of welfare.
Welfare of aquatic species is at the heart of the fair-fish database. In our definition of welfare, we follow Broom (1986): “The welfare of an individual is its state as regards its attempts to cope with its environment.” Thus, welfare may be perceived as a continuum on which an individual rates “good” or “poor” or everything in between.
We pursue what could be called a combination of not only a) valuing the freedom from injuries and stress (function-based approach) but b) supporting attempts to provide rewarding experiences and cognitive challenges (feelings-based approach) as well as c) arguing for enclosures that mimic the wild habitat as best as possible and allow for natural behaviour (nature-based approach).
Try mousing over the element you are interested in - oftentimes you will find explanations this way. If not, there will be FAQ on many of the sub-pages with answers to questions that apply to the respective sub-page. If your question is not among those, contact us at ffdb@fair-fish.net.
It's right here! We decided to re-name it to fair-fish database for several reasons. The database has grown beyond dealing purely with ethology, more towards welfare in general – and so much more. Also, the partners fair-fish and FishEthoGroup decided to re-organise their partnership. While maintaining our friendship, we also desire for greater independence. So, the name "fair-fish database" establishes it as a fair-fish endeavour.