Information
Authors: María J. Cabrera-Álvarez, Maria Filipa Castanheira
Version: C | 1.1Published: 2024-12-31
- minor editorial changes plus new side note "Commercial relevance"
- profile update resulting in major editorial and content changes (changing the scoring in criteria 1-3, 5-10)
- transfer to consistent age class and label structure resulting in changed appearance
WelfareScore | farm
The score card gives our welfare assessments for aquatic species in 10 criteria.
For each criterion, we score the probability to experience good welfare under minimal farming conditions ("Likelihood") and under high-standard farming conditions ("Potential") representing the worst and best case scenario. The third dimension scores how certain we are of our assessments based on the number and quality of sources we found ("Certainty").
The WelfareScore sums just the "High" scores in each dimension. Although good welfare ("High") seems not possible in some criteria, there could be at least a potential improvement from low to medium welfare (indicated by ➚ and the number of criteria).
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
➚ = potential improvements not reaching "High" - Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10 per dimension)
General remarks
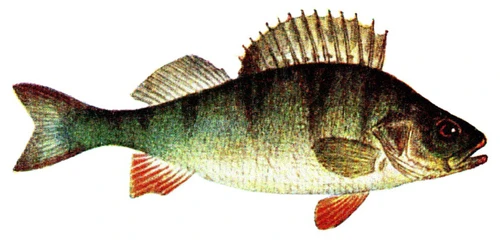
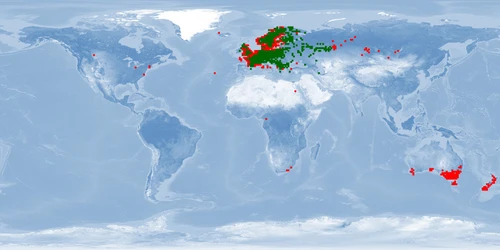
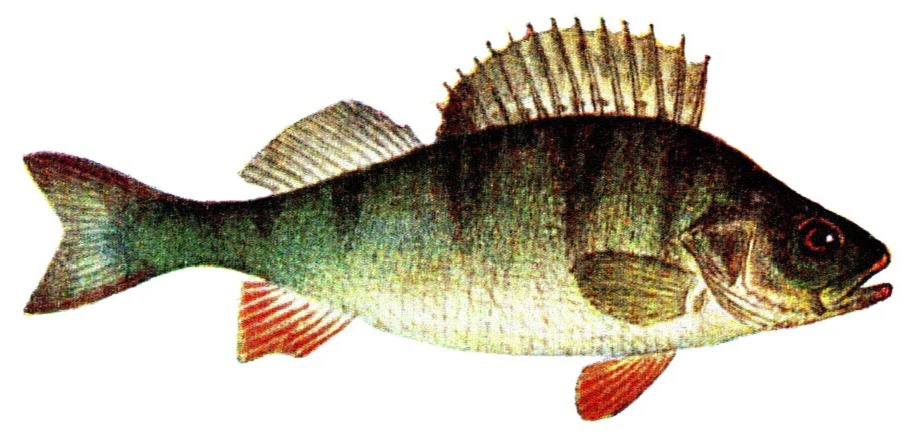
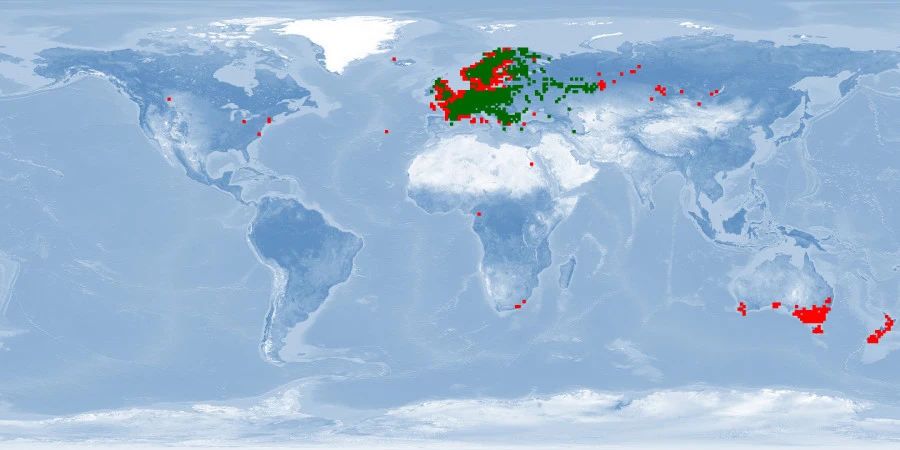
Perca fluviatilis is a percid species that inhabits Eurasian inland and coastal waters and has been introduced in inland waters worldwide. The production of P. fluviatilis has emerged over the past decades while important biological processes of the species are not known yet. P. fluviatilis is a strong predator in the wild, and as such, an aggressive and cannibalistic fish in captivity – a constraint that is not properly prevented in farms yet. In fact, prey FISHES (e.g., roach, Rutilus rutilus, topmouth gudgeon, Pseudorasbora parva or other small cyprinids species) are added in monoculture systems to satiate the predatory nature of P. fluviatilis. It is also susceptible to stress and malformations in captivity. Tanks or raceways will most likely not fulfil space needs in intensive conditions. The biggest knowledge gap is on humane slaughter practices for this species. Further research is needed on both natural behaviour and physiological effects of farming practices in order to provide recommendations for improving fish welfare.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
It is low for minimal farming conditions, as tanks, RAS, and some ponds do not cover the whole range in the wild. It is medium for high-standard farming conditions, as other ponds at least overlap with the range in the wild, although we cannot be sure in most age classes. Our conclusion is based on a medium amount of evidence, as further wild information is missing in LARVAE, FRY, and JUVENILES.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal farming conditions, as cages, ponds, and tanks do not cover the whole range in the wild. It is medium for high-standard farming conditions, as ponds overlap with the range in the wild. Our conclusion is based on a high amount of evidence.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal farming conditions, as the ANADROMOUS strain undertakes more or less extensive migrations, and we cannot be sure that providing each age class with their respective environmental conditions will satisfy their urge to migrate or whether they need to experience the transition. It is high for high-standard farming conditions given the resident strain. Our conclusion is based on a medium amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of these circumstances?
It is low for minimal farming conditions, as the species is manipulated (hormonal manipulation, stripping) and may be taken from the wild. It is high for high-standard farming conditions, as natural breeding with farm-reared IND is possible and verified for the farming context. Our conclusion is based on a medium amount of evidence, as further research is needed on reproduction behaviour in the wild.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is low for minimal farming conditions, as densities in tanks and some ponds go beyond the minimum density in the wild. It is high for high-standard farming conditions, as densities in other ponds potentially cover the range in the wild. Our conclusion is based on a medium amount of evidence.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal farming conditions, as the species is aggressive – even cannibalistic – in almost all age classes. It is medium for high-standard farming conditions, as ways to reduce (but not avoid) cannibalism (size homogeneity, reducing densities) are verified for the farming context. Our conclusion is based on a high amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions, as almost all age classes of the species use substrate, but RAS and some ponds are devoid of it. It is high for high-standard farming conditions given a) hatching substrate for eggs, b) earthen ponds for JUVENILES and ADULTS which are not lined, and given b) natural reproduction with spawning substrate in ponds for SPAWNERS. Our conclusion is based on a high amount of evidence.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions, as the species is stressed (confinement, handling, husbandry). It is medium for high-standard farming conditions, as improvements are easily imaginable, but need to be verified for the farming context. Our conclusion is based on a medium amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is low for minimal farming conditions, as malformation rates may exceed 10%. It is medium for high-standard farming conditions, as some malformations result from conditions that may be changed (rearing environment, feed). Our conclusion is based on a medium amount of evidence, as improvement of the situation by adjusting conditions needs more proof.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is unclear for minimal and high-standard farming conditions, although percussive stunning followed by bleeding seems promising, but needs to be verified for the farming context. Our conclusion is based on a low amount of evidence.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 4 102, level 5 being fully domesticated. Cultured since 1950 103.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes:
- WILD: carnivorous and piscivorous, mainly zooplankton as JUVENILES, increasing proportion of fish with increasing age 49 26 32 82.
- FARM: limited application of fish oil replacements due to alterations in liver structure 104. JUVENILES: fish meal may be not 105 to partly* replaced by non-forage fishery components 106.
- LAB: FINGERLINGS: fish meal may be not replaced by sustainable sources 107. JUVENILES: fish meal may be partly* replaced by non-forage fishery components 108 109 110.
* partly = <51% – mostly = 51-99% – completely = 100%
Side note: Commercial relevance
How much is this species farmed annually?
954 t/year 1990-2019 amounting to estimated 8,000,000-12,000,000 IND/year 1990-2019 111.
Glossary
ANADROMOUS = migrating from the sea into fresh water to spawn
DOMESTICATION LEVEL 4 = entire life cycle closed in captivity without wild inputs 102
EURYHALINE = tolerant of a wide range of salinities
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FINGERLINGS = early juveniles with fully developed scales and working fins, the size of a human finger
FISHES = using "fishes" instead of "fish" for more than one individual - whether of the same species or not - is inspired by Jonathan Balcombe who proposed this usage in his book "What a fish knows". By referring to a group as "fishes", we acknowledge the individuals with their personalities and needs instead of an anonymous mass of "fish".
FRY = larvae from external feeding on
IND = individuals
JUVENILES = fully developed but immature individuals
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening
PELAGIC = living independent of bottom and shore of a body of water
PHOTOPERIOD = duration of daylight
RAS = Recirculating Aquaculture System - almost completely closed system using filters to clean and recirculate water with the aim of reducing water input and with the advantage of enabling close control of environmental parameters to maintain high water quality
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
TOTAL LENGTH = from snout to tip of caudal fin as compared to fork length (which measures from snout to fork of caudal fin) or standard length (from head to base of tail fin) or body length (from the base of the eye notch to the posterior end of the telson) 76
WILD = setting in the wild
Bibliography
2 Kestemont, P., C. Mélard, J. A. Held, and K. Dabrowski. 2015. Culture Methods of Eurasian Perch and Yellow Perch Early Life Stages. In Biology and Culture of Percid Fishes, ed. Patrick Kestemont, Konrad Dabrowski, and Robert C. Summerfelt, 265–293. Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-94-017-7227-3_9.
3 Kestemont, Patrick, Carole Rougeot, Jiri Musil, and Damien Toner. 2008. Larval and Juvenile Production. In Farming of Eurasian Perch, ed. Damien Toner and Carole Rougeot, 1-Juvenile Production:30–41. Aquaculture Explained 24. Dublin: Aquaculture Development Division, Bord Iascaigh.
4 Lahnsteiner, Franz, and Manfred Kletzl. 2018. A method for Rearing Perch, Perca fluviatilis, Larvae Using Paramecium caudatum, Followed by Wild Zooplankton and Formulated Dry Feed in Combination With Adequate Tank Systems. Journal of Agricultural Science 10: 26. https://doi.org/10.5539/jas.v10n8p26.
5 Jacquemond, François. 2004. Separated breeding of perch fingerlings (Perca fluviatilis L.) with and without initial inflated swim bladder: comparison of swim bladder development, skeleton conformation and growth performances. Aquaculture 239: 261–273. https://doi.org/10.1016/j.aquaculture.2004.06.019.
6 Jankowska, Barbara, Zdzisław Zakęś, Tomasz Żmijewski, Szczepkowski Mirosław, and Agata Cejko. 2007. Slaughter yield, proximate composition, and flesh colour of cultivated and wild perch (Perca fluviatilis L.). Czech Journal of Animal Science 52: 260–267. https://doi.org/10.17221/2279-CJAS.
7 Policar, Tomas, Damien Toner, S.M.H. Alavi, and Ottomar Linhart. 2008. Reproduction and Spawning. In Farming of Eurasian Perch, ed. Damien Toner and Carole Rougeot, 1-Juvenile Production:22–29. Aquaculture Explained 24. Dublin: Aquaculture Development Division, Bord Iascaigh.
8 Policar, Tomáš, Azin Mohagheghi Samarin, and Charles Mélard. 2015. Culture Methods of Eurasian Perch During Ongrowing. In Biology and Culture of Percid Fishes, ed. Patrick Kestemont, Konrad Dabrowski, and Robert C. Summerfelt, 417–435. Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-94-017-7227-3_16.
9 Eklöv, P. 1997. Effects of habitat complexity and prey abundance on the spatial and temporal distributions of perch (Perca fluviatilis) and pike (Esox lucius). Canadian Journal of Fisheries and Aquatic Sciences 54: 1520–1531. https://doi.org/10.1139/cjfas-54-7-1520.
10 Linløkken, Arne, Eva Bergman, Larry Greenberg, and Per Arne Holt Seeland. 2008. Environmental correlates of population variables of perch (Perca fluviatilis) in boreal lakes. Environmental Biology of Fishes 82: 401–408. https://doi.org/10.1007/s10641-007-9301-y.
11 Francová, Kateřina, and Markéta Ondračková. 2014. Effect of habitat conditions on parasite infection in 0+ juvenile perch (Perca fluviatilis L.) in two Czech reservoirs. Hydrobiologia 721. https://doi.org/10.1007/s10750-013-1644-0.
12 Bochert, Ralf. 2022. Comparative performance, biochemical composition, and fatty acid analysis of Eurasian perch (Perca fluviatilis) during grow-out in RAS fed different commercial diets. Journal of Applied Aquaculture 34: 208–222. https://doi.org/10.1080/10454438.2020.1828217.
13 Monk, Christopher T., and Robert Arlinghaus. 2017. Eurasian perch, Perca fluviatilis, spatial behaviour determines vulnerability independent of angler skill in a whole-lake reality mining experiment. Canadian Journal of Fisheries and Aquatic Sciences 75: 417–428. https://doi.org/10.1139/cjfas-2017-0029.
14 Westrelin, Samuel, Romain Roy, Laurence Tissot-Rey, Laurent Bergès, and Christine Argillier. 2018. Habitat use and preference of adult perch (Perca fluviatilis L.) in a deep reservoir: variations with seasons, water levels and individuals. Hydrobiologia 809: 121–139. https://doi.org/10.1007/s10750-017-3454-2.
15 Fontaine, Pascal, Patrick Kestemont, and Charles Mélard. 2008. Broodstock Management. In Farming of Eurasian Perch, ed. Damien Toner and Carole Rougeot, 1-Juvenile Production:16–21. Aquaculture Explained 24. Dublin: Aquaculture Development Division, Bord Iascaigh.
16 Křišťan, Jiří, Vlastimil Stejskal, and Tomáš Policar. 2012. Comparison of Reproduction Characteristics and Broodstock Mortality in Farmed and Wild Eurasian Perch (Perca fluviatilis L.) Females During Spawning Season Under Controlled Conditions. Turkish Journal of Fisheries and Aquatic Sciences 12: 191–197.
17 Steenfeldt, Svend, Pascal Fontaine, Julia Lynne Overton, Tomáš Policar, Damien Toner, Bahram Falahatkar, Ákos Horváth, Ines Ben Khemis, Neila Hamza, and Mohammed Mhetli. 2015. Current Status of Eurasian Percid Fishes Aquaculture. In Biology and Culture of Percid Fishes: Principles and Practices, ed. Patrick Kestemont, Konrad Dabrowski, and Robert C. Summerfelt, 817–841. Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-94-017-7227-3_32.
18 Skovrind, Mikkel, Emil A. F. Christensen, Henrik Carl, Lene Jacobsen, and Peter R. Møller. 2013. Marine spawning sites of perch Perca fluviatilis revealed by oviduct-inserted acoustic transmitters. Aquatic Biology 19: 201–206. https://doi.org/10.3354/ab00529.
19 Thorpe, John. 1977. Synopsis of biological data on the perch: Perca fluviatilis Linnaeus, 1758 and Perca flavescens Mitchill, 1814. FAO Fisheries Synopsis 113. Rome, Italy: Food and Agriculture Organization of the United Nations.
20 Čech, Martin, Jiří Peterka, Milan Říha, Vladislav Draštík, Michal Kratochvíl, and Jan Kubečka. 2010. Deep spawning of perch (Perca fluviatilis, L.) in the newly created Chabařovice Lake, Czech Republic. Hydrobiologia 649: 375–378. https://doi.org/10.1007/s10750-010-0251-6.
21 Viljanen, Markku, and Ismo J. Holopainen. 1982. Population density of perch (Perca fluviatilis L.) at egg, larval and adult stages in the dys-oligotrophic Lake Suomunjärvi, Finland. Annales Zoologici Fennici 19: 39–46.
22 Treasurer, James W. 1983. Estimates of egg and viable embryo production in a lacustrine perch, Perca fluviatilis. Environmental Biology of Fishes 8: 3–16. https://doi.org/10.1007/BF00004941.
23 Gillet, C., and J. P. Dubois. 1995. A survey of the spawning of perch (Perca fluviatilis), pike (Esox lucius), and roach (Rutilus rutilus), using artificial spawning substrates in lakes. In Space Partition within Aquatic Ecosystems, ed. Gérard Balvay, 409–415. Developments in Hydrobiology 104. Springer Netherlands. https://doi.org/10.1007/978-94-011-0293-3_39.
24 Dubois, Jean-Paul, Christian Gillet, Stéphane Bonnet, and Yvette Chevalier-Weber. 1996. Correlation between the size of mature female perch (Perca fluviatilis L.) and the width of their egg strands in Lake Geneva. Annales Zoologici Fennici 33: 417–420.
25 Probst, W. N., S. Stoll, H. Hofmann, P. Fischer, and R. Eckmann. 2009. Spawning site selection by Eurasian perch (Perca fluviatilis L.) in relation to temperature and wave exposure. Ecology of Freshwater Fish 18: 1–7. https://doi.org/10.1111/j.1600-0633.2008.00327.x.
26 Persson, Lennart, Pär Byström, and Eva Wahlström. 2000. Cannibalism and Competition in Eurasian Perch: Population Dynamics of an Ontogenetic Omnivore. Ecology 81: 1058–1071. https://doi.org/10.1890/0012-9658(2000)081[1058:CACIEP]2.0.CO;2.
27 Petrtýl, Miloslav, Lukáš Kalous, Jaroslava Frouzová, and Martin Čech. 2015. Effects of habitat type on short- and long-term growth parameters of the European perch (Perca fluviatilis L.): Size distribution and RNA/DNA ratio of European perch fry. International Review of Hydrobiology 100: 13–20. https://doi.org/10.1002/iroh.201301675.
28 Čech, M., J. Kubečka, J. Frouzová, V. Draštík, M. Kratochvíl, J. Matěna, and J. Hejzlar. 2007. Distribution of the bathypelagic perch fry layer along the longitudinal profile of two large canyon-shaped reservoirs. Journal of Fish Biology 70: 141–154. https://doi.org/10.1111/j.1095-8649.2006.01282.x.
29 Craig, John F. 1977. Seasonal changes in the day and night activity of adult perch, Perca fluviatilis L. Journal of Fish Biology 11: 161–166.
30 Jellyman, D. J. 1980. Age, growth, and reproduction of perch, Perca fluviatilis L., in Lake Pounui. New Zealand Journal of Marine and Freshwater Research 14: 391–400. https://doi.org/10.1080/00288330.1980.9515881.
31 Egloff, Mathias. 1996. Failure of swim bladder inflation of perch, Perca fluviatilis L. found in natural populations. Aquatic Sciences 58: 15–23.
32 Ceccuzzi, Pietro, Genciana Terova, Fabio Brambilla, Micaela Antonini, and Marco Saroglia. 2011. Growth, diet, and reproduction of Eurasian perch Perca fluviatilis L. in Lake Varese, northwestern Italy. Fisheries Science 77: 533–545. https://doi.org/10.1007/s12562-011-0353-8.
33 Ferter, Keno, and Victor Benno Meyer-Rochow. 2010. Turning Night into Day: Effects of Stress on the Self-Feeding Behaviour of the Eurasian Perch Perca fluviatilis. Zoological Studies 49: 176–181.
34 Allen, K. R. 1935. The Food and Migration of the Perch (Perca fluviatilis) in Windermere. Journal of Animal Ecology 4: 264–273. https://doi.org/10.2307/1016.
35 Čech, M., J. Peterka, M. Říha, L. Vejřík, T. Jůza, M. Kratochvíl, V. Draštík, M. Muška, P. Znachor, and J. Kubečka. 2012. Extremely shallow spawning of perch (Perca fluviatilis L.): the roles of sheltered bays, dense semi-terrestrial vegetation and low visibility in deeper water. Knowledge and Management of Aquatic Ecosystems 406: 12. https://doi.org/10.1051/kmae/2012026.
36 Cabrera-Álvarez, María. 2023. Conclusion.
37 Froese, R., and D. Pauly. 2017. Perca fluviatilis summary page. World Wide Web electronic publication. www.fishbase.org.
38 Kratochvil, M., J. Peterka, J. Kubecka, J. Matena, M. Vasek, I. Vanickova, M. Cech, and Jaromir Seda. 2008. Diet of larvae and juvenile perch, Perca fluviatilis performing diel vertical migrations in a deep reservoir. Folia Zoologica 57: 313–323.
39 Craig, J. F. 1974. Population dynamics of perch, Perca fluviatilis L. in Slapton Ley, Devon. I. Trapping behaviour, reproduction, migration, population estimates, mortality and food. Freshwater Biology 4: 417–431. https://doi.org/10.1111/j.1365-2427.1974.tb00106.x.
40 Le Cren, E. D. 1958. Observations on the Growth of Perch (Perca fluviatilis L.) Over Twenty-Two Years with Special Reference to the Effects of Temperature and Changes in Population Density. Journal of Animal Ecology 27: 287–334. https://doi.org/10.2307/2242.
41 Komolka, Katrin, Ralf Bochert, George P. Franz, Yagmur Kaya, Ralf Pfuhl, and Bianka Grunow. 2020. Determination and Comparison of Physical Meat Quality Parameters of Percidae and Salmonidae in Aquaculture. Foods 9: 388. https://doi.org/10.3390/foods9040388.
42 Järv, Leili. 2000. Migration of the perch (Perca fluviatilis L.) in the coastal waters of Western Estonia. In Proceedings of the Estonian Academy of Sciences, Biology and Ecology. Estonian Academy Publishers.
43 Järv, L. 2000. Perch Perca fluviatilis L. in Estonian coastal waters. Science Biology Ecology 49. Proc. Estonian Acad.: 270–276.
44 Gerlach, Gabriele, Uwe Schardt, Reiner Eckmann, and Axel Meyer. 2001. Kin-structured subpopulations in Eurasian perch (Perca fluviatilis L.). Heredity 86: 213–221.
45 Nesbø, C. L., C. Magnhagen, and K. S. Jakobsen. 1998. Genetic Differentiation Among Stationary and Anadromous Perch (Perca Fluviatilis) in the Baltic Sea. Hereditas 129: 241–249. https://doi.org/10.1111/j.1601-5223.1998.00241.x.
46 Snickars, Martin, Göran Sundblad, Alfred Sandström, Lars Ljunggren, Ulf Bergström, Gustav Johansson, and Johanna Mattila. 2010. Habitat selectivity of substrate-spawning fish: Modeling requirements for the Eurasian perch Perca fluviatilis. Marine Ecology Progress Series 398: 235–243. https://doi.org/10.3354/meps08313.
47 Tibblin, Petter, Per Koch-Schmidt, Per Larsson, and Patrik Stenroth. 2012. Effects of salinity on growth and mortality of migratory and resident forms of Eurasian perch in the Baltic Sea. Ecology of Freshwater Fish 21: 200–206. https://doi.org/10.1111/j.1600-0633.2011.00537.x.
48 Treasurer, J. W. 1981. Some aspects of the reproductive biology of perch Perca fluviatilis L. Fecundity, maturation and spawning behaviour. Journal of Fish Biology 18: 729–740. https://doi.org/10.1111/j.1095-8649.1981.tb03814.x.
49 Rask, Martti. 1983. Differences in growth of perch (Perca fluviatilis L.) in two small forest lakes. Hydrobiologia 101: 139–143. https://doi.org/10.1007/BF00008666.
50 Saemi Komsari, M., A. Bani, H. Khara, and H. Reza Esmaeili. 2014. Reproductive strategy of the European perch, Perca fluviatilis Linnaeus, 1758 (Osteichthyes: Percidae) in the Anzali wetland, southwest Caspian Sea. Journal of Applied Ichthyology 30: 307–313. https://doi.org/10.1111/jai.12335.
51 Voskoboinikova, O. S., and I. G. Grechanov. 2002. Development of the Skeleton during the Ontogenesis of the River Perch Perca fluviatilis. Journal of Ichthyology 42: 322–333.
52 Čech, Martin, Lukáš Vejřík, Jiří Peterka, Milan Říha, Milan Muška, Tomáš Jůza, Vladislav Draštík, Michal Kratochvíl, and Jan Kubečka. 2012. The use of artificial spawning substrates in order to understand the factors influencing the spawning site selection, depth of egg strands deposition and hatching time of perch (Perca fluviatilis L.). J. Limnol. 71: 18. https://doi.org/10.4081/jlimnol.2012.e18.
53 Hokanson, Kenneth E. F. 1977. Temperature Requirements of Some Percids and Adaptations to the Seasonal Temperature Cycle. Journal of the Fisheries Research Board of Canada 34: 1524–1550. https://doi.org/10.1139/f77-217.
54 Thorpe, J. E. 1977. Morphology, Physiology, Behavior, and Ecology of Perca fluviatilis L. and P. flavescens Mitchill. Journal of the Fisheries Research Board of Canada 34: 1504–1514. https://doi.org/10.1139/f77-215.
55 Craig, John F. 2000. Percid Fishes: Systematics, Ecology and Exploitation. John Wiley & Sons.
56 CABI. 2021. Perca fluviatilis (perch). CABI Compendium CABI Compendium: 70037. https://doi.org/10.1079/cabicompendium.70037.
57 Kucharczyk, D, R Kujawa, A Mamcarz, A Skrzypczak, and E Wyszomirska. 1998. Induced spawning in perch, Perca fluviatilis L., using FSH + LH with pimozide or metoclopramide. Aquaculture Research 29: 131–136. https://doi.org/10.1046/j.1365-2109.1998.00949.x.
58 Kouril, J., and J. Hamackova. 2000. The semiartificial and artificial hormonally induced propagation of European perch (Perca fluviatilis). In Proc. Aqua, 2000. Responsible Aquaculture in the New Millennium, ed. R. Floss and L. Creswell, 28:345. Spec. Publ. Oostende, Belgium.
59 Żarski, D., A. Horváth, J. A. Held, and D. Kucharczyk. 2015. Artificial Reproduction of Percid Fishes. In Biology and Culture of Percid Fishes - Principles and Practices, ed. P. Kestemont, K. Dabrowski, and Robert C. Summerfelt. Dordrecht: Springer Netherlands.
60 Rónyai, András, and Svetlana Anatolyevna Lengyel. 2010. Effects of hormonal treatments on induced tank spawning of Eurasian perch (Perca fluviatilis L.). Aquaculture Research 41: e345–e347. https://doi.org/10.1111/j.1365-2109.2009.02465.x.
61 Żarski, Daniel, Zoltán Bokor, László Kotrik, Béla Urbanyi, Akos Horváth, Katarzyna Targońska, Sławomir Krejszeff, Katarzyna Palińska, and Dariusz Kucharczyk. 2011. A new classification of a preovulatory oocyte maturation stage suitable for the synchronization of ovulation in controlled reproduction of Eurasian perch, Perca fluviatilis L. Reproductive Biology 11: 194–209. https://doi.org/10.1016/S1642-431X(12)60066-7.
62 Rougeot, Carole. 2015. Sex and Ploidy Manipulation in Percid Fishes. In Biology and Culture of Percid Fishes: Principles and Practices, ed. Patrick Kestemont, Konrad Dabrowski, and Robert C. Summerfelt, 625–635. Dordrecht: Springer Netherlands.
63 Behrmann-Godel, Jasminca, Gabriele Gerlach, and Reiner Eckmann. 2006. Kin and Population Recognition in Sympatric Lake Constance Perch (Perca fluviatilis L.): Can Assortative Shoaling Drive Population Divergence? Behavioral Ecology and Sociobiology 59: 461–468.
64 Magnhagen, Carin. 2015. Behaviour of Percid Fishes in the Wild and Its Relevance for Culture. In Biology and Culture of Percid Fishes: Principles and Practices, ed. Patrick Kestemont, Konrad Dabrowski, and Robert C. Summerfelt, 399–417. Dordrecht: Springer Netherlands.
65 Guillard, Jean, Patrice Brehmer, Michel Colon, and Yvon Guennégan. 2006. Three dimensional characteristics of young–of–year pelagic fish schools in lake. Aquatic Living Resources 19: 115–122. https://doi.org/10.1051/alr:2006011.
66 Čech, M., M. Kratochvíl, J. Kubečka, V. Draštík, and J. Matěna. 2005. Diel vertical migrations of bathypelagic perch fry. Journal of Fish Biology 66: 685–702. https://doi.org/10.1111/j.0022-1112.2005.00630.x.
67 Magnhagen, Carin, and Nils Bunnefeld. 2009. Express your personality or go along with the group: what determines the behaviour of shoaling perch? Proceedings of the Royal Society of London B: Biological Sciences 276: 3369–3375. https://doi.org/10.1098/rspb.2009.0851.
68 Probst, Wolfgang Nikolaus, Gregor Thomas, and Reiner Eckmann. 2009. Hydroacoustic observations of surface shoaling behaviour of young-of-the-year perch Perca fluviatilis (Linnaeus, 1758) with a towed upward-facing transducer. Fisheries Research 96: 133–138. https://doi.org/10.1016/j.fishres.2008.10.009.
69 Overton, JL, and H Paulsen. 2005. Ongrowing of Perch (Perca fluviatilis) Juveniles (Videreopdræt af aborreyngel). No. 151-05. Danmarks Fiskeriundersøgelser, Afdeling for Havøkologi og Akvakultur, Bornholms Lakseklækkeri.
70 Janssens, Thomas. 2017. Personal communication.
71 Strand, Å., A. Alanärä, and C. Magnhagen. 2007. Effect of group size on feed intake, growth and feed efficiency of juvenile perch. Journal of Fish Biology 71: 615–619. https://doi.org/10.1111/j.1095-8649.2007.01497.x.
72 Schleuter, Diana, Susanne Haertel-Borer, Philipp Fischer, and Reiner Eckmann. 2007. Respiration Rates of Eurasian Perch Perca fluviatilis and Ruffe: Lower Energy Costs in Groups. Transactions of the American Fisheries Society 136: 43–55. https://doi.org/10.1577/T06-123.1.
73 Bruylants, B., A. Vandelannoote, and R. Verheyen. 1986. The movement pattern and density distribution of perch, Perca fluviatilis L., in a channelized lowland river. Aquaculture Research 17: 49–57. https://doi.org/10.1111/j.1365-2109.1986.tb00084.x.
74 Le Cren, E. D. 1992. Exceptionally big individual perch (Perca fluviatilis L.) and their growth. Journal of Fish Biology 40: 599–625. https://doi.org/10.1111/j.1095-8649.1992.tb02609.x.
75 Hematyar, Nima, Jan Mraz, Vlastimil Stejskal, Sabine Sampels, Zuzana Linhartová, Marketa Prokesova, Frantisek Vacha, et al. 2021. Comparison of Quality Changes in Eurasian Perch (Perca fluviatilis L.) Fillets Originated from Two Different Rearing Systems during Frozen and Refrigerated Storage. Foods 10: 1405. https://doi.org/10.3390/foods10061405.
76 Pawson, M.G., and G.D. Pickett. 1996. The Annual Pattern of Condition and Maturity in Bass, Dicentrarchus Labrax, in Waters Around England and Wales. Journal of the Marine Biological Association of the United Kingdom 76: 107. https://doi.org/10.1017/S0025315400029040.
77 Baras, Etienne, Patrick Kestemont, and Charles Mélard. 2003. Effect of stocking density on the dynamics of cannibalism in sibling larvae of Perca fluviatilis under controlled conditions. Aquaculture 219: 241–255. https://doi.org/10.1016/S0044-8486(02)00349-6.
78 Król, Jaroslaw, Nicolas Dauchot, Syaghalirwa N M Mandiki, Pierre van Cutsem, and Patrick Kestemont. 2015. Cannibalism in cultured eurasian perch, Perca fluviatilis (Actinopterygii: Perciformes: Percidae)-Implication of maternal influence, kinship, and sex ratio of progenies. Acta Ichthyologica et Piscatoria 45: 65–73. https://doi.org/10.3750/AIP2015.45.1.07.
79 Westerberg, Magdalena, Fia Staffan, and Carin Magnhagen. 2004. Influence of predation risk on individual competitive ability and growth in Eurasian perch, Perca fluviatilis. Animal Behaviour 67: 273–279. https://doi.org/10.1016/j.anbehav.2003.06.003.
80 Magnhagen, C., and E. Heibo. 2004. Growth in length and in body depth in young-of-the-year perch with different predation risk. Journal of Fish Biology 64: 612–624. https://doi.org/10.1111/j.1095-8649.2004.00325.x.
81 Magnhagen, Carin, and Jost Borcherding. 2008. Risk-taking behaviour in foraging perch: does predation pressure influence age-specific boldness? Animal Behaviour 75: 509–517. https://doi.org/10.1016/j.anbehav.2007.06.007.
82 Heermann, Lisa, Werner Scharf, Gerard van der Velde, and Jost Borcherding. 2014. Does the use of alternative food resources induce cannibalism in a size-structured fish population? Ecology of Freshwater Fish 23: 129–140. https://doi.org/10.1111/eff.12060.
83 Mikheev, Victor N., Anna F. Pasternak, Gerhard Tischler, and Josef Wanzenböck. 2005. Contestable shelters provoke aggression among 0+ perch, Perca fluviatilis. Environmental Biology of Fishes 73: 227–231. https://doi.org/10.1007/s10641-005-0558-8.
84 Goldenberg, Silvan U., Jost Borcherding, and Martina Heynen. 2014. Balancing the response to predation—the effects of shoal size, predation risk and habituation on behaviour of juvenile perch. Behavioral Ecology and Sociobiology 68: 989–998. https://doi.org/10.1007/s00265-014-1711-1.
85 Staffan, F., C. Magnhagen, and A. Alanärä. 2002. Variation in food intake within groups of juvenile perch. Journal of Fish Biology 60: 771–774. https://doi.org/10.1111/j.1095-8649.2002.tb01702.x.
86 Treasurer, J. W., and F. G. T. Holliday. 1981. Some aspects of the reproductive biology of perch Perca fluviatilis L. A histological description of the reproductive cycle. Journal of Fish Biology 18: 359–376. https://doi.org/10.1111/j.1095-8649.1981.tb03778.x.
87 Jentoft, Sissel, Sigurd ØXnevad, Are H. Aastveit, and ØIvind Andersen. 2006. Effects of Tank Wall Color and Up-welling Water Flow on Growth and Survival of Eurasian Perch Larvae (Perca fluviatilis). Journal of the World Aquaculture Society 37: 313–317. https://doi.org/10.1111/j.1749-7345.2006.00042.x.
88 Borcherding, Jost. 2006. Prey or predator: 0+ perch (Perca fluviatilis) in the trade-off between food and shelter. Environmental Biology of Fishes 77: 87–96. https://doi.org/10.1007/s10641-006-9057-9.
89 Strand, Å., A. Alanärä, F. Staffan, and C. Magnhagen. 2007. Effects of tank colour and light intensity on feed intake, growth rate and energy expenditure of juvenile Eurasian perch, Perca fluviatilis L. Aquaculture 272: 312–318. https://doi.org/10.1016/j.aquaculture.2007.08.052.
90 Douxfils, J., S. N. M. Mandiki, G. Marotte, N. Wang, F. Silvestre, S. Milla, E. Henrotte, et al. 2011. Does domestication process affect stress response in juvenile Eurasian perch Perca fluviatilis? Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 159: 92–99. https://doi.org/10.1016/j.cbpa.2011.01.021.
91 Haux, Carl, Maj-Lis Sjöbeck, and Åke Larsson. 1985. Physiological stress responses in a wild fish population of perch (Perca fluviatilis) after capture and during subsequent recovery. Marine Environmental Research 15: 77–95. https://doi.org/10.1016/0141-1136(85)90131-X.
92 Acerete, L, J. C Balasch, E Espinosa, A Josa, and L Tort. 2004. Physiological responses in Eurasian perch (Perca fluviatilis, L.) subjected to stress by transport and handling. Aquaculture 237: 167–178. https://doi.org/10.1016/j.aquaculture.2004.03.018.
93 Jentoft, Sissel, Are H. Aastveit, Peter A. Torjesen, and Øivind Andersen. 2005. Effects of stress on growth, cortisol and glucose levels in non-domesticated Eurasian perch (Perca fluviatilis) and domesticated rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 141: 353–358. https://doi.org/10.1016/j.cbpb.2005.06.006.
94 Milla, Sylvain, Cédric Mathieu, Neil Wang, Sophie Lambert, Stéphanie Nadzialek, Sophie Massart, Emilie Henrotte, et al. 2010. Spleen immune status is affected after acute handling stress but not regulated by cortisol in Eurasian perch, Perca fluviatilis. Fish & Shellfish Immunology 28: 931–941. https://doi.org/10.1016/j.fsi.2010.02.012.
95 Douxfils, J., S. Lambert, C. Mathieu, S. Milla, S. N. M. Mandiki, E. Henrotte, N. Wang, et al. 2014. Influence of domestication process on immune response to repeated emersion stressors in Eurasian perch (Perca fluviatilis, L.). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 173: 52–60. https://doi.org/10.1016/j.cbpa.2014.03.012.
96 Alanärä, A., and Å. Strand. 2015. The Energy Requirements of Percid Fish in Culture. In Biology and Culture of Percid Fishes: Principles and Practices, ed. P. Kestemont, K. Dabrowski, and Robert C. Summerfelt, 353–369. Dordrecht: Springer Netherlands.
97 Brüning, Anika, Franz Hölker, Steffen Franke, Torsten Preuer, and Werner Kloas. 2015. Spotlight on fish: Light pollution affects circadian rhythms of European perch but does not cause stress. Science of The Total Environment 511: 516–522. https://doi.org/10.1016/j.scitotenv.2014.12.094.
98 Wysocki, Lidia Eva, John P. Dittami, and Friedrich Ladich. 2006. Ship noise and cortisol secretion in European freshwater fishes. Biological Conservation 128: 501–508. https://doi.org/10.1016/j.biocon.2005.10.020.
99 Strand, Å., C. Magnhagen, and A. Alanärä. 2007. Effects of repeated disturbances on feed intake, growth rates and energy expenditures of juvenile perch, Perca fluviatilis. Aquaculture 265: 163–168. https://doi.org/10.1016/j.aquaculture.2007.01.030.
100 Douxfils, Jessica, S. N. M Mandiki, C. Mathieu, S. Milla, and M. Saroglia. 2015. Domestication and Responses to Stress. In Biology and Culture of Percid Fishes: Principles and Practices, ed. P. Kestemont, K. Dabrowski, and Robert C. Summerfelt, 743–761. Dordrecht: Springer Netherlands.
101 Alix, M., D. Zarski, D. Chardard, P. Fontaine, and B. Schaerlinger. 2017. Deformities in newly hatched embryos of Eurasian perch populations originating from two different rearing systems. Journal of Zoology 302: 126–137. https://doi.org/10.1111/jzo.12447.
102 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
103 FAO. 2017. FAO Fisheries & Aquaculture - Species Fact Sheets - Perca fluviatilis (Linnaeus, 1758). World Wide Web electronic publication. www.fao.org.
104 Geay, Florian, and Patrick Kestemont. 2015. Feeding and Nutrition of Percid Fishes During Ongrowing Stages. In Biology and Culture of Percid Fishes: Principles and Practices, ed. Patrick Kestemont, Konrad Dabrowski, and Robert C. Summerfelt, 587–625. Dordrecht: Springer Netherlands.
105 Tilami, Sarvenaz Khalili, Jan Turek, Daniel Červený, Pavel Lepič, Pavel Kozák, Viktoriia Burkina, Sidika Sakalli, Aleš Tomčala, Sabine Sampels, and Jan Mráz. 2020. Insect Meal as a Partial Replacement for Fish Meal in a Formulated Diet for Perch Perca fluviatilis. Turkish Journal of Fisheries and Aquatic Sciences 20: 867–878. https://doi.org/10.4194/1303-2712-v20_12_03.
106 Tran, Hung Quang, Elena Wernicke von Siebenthal, Jean-Baptiste Luce, Tram Thi Nguyen, Aleš Tomčala, Vlastimil Stejskal, and Thomas Janssens. 2024. Complementarity of insect meal and poultry by-product meal as replacement for fishmeal can sustain the production performance of European perch (Perca fluviatilis), reduce economic fish-in fish-out ratio and food-feed competition, and influence the environmental indices. Aquaculture 579: 740166. https://doi.org/10.1016/j.aquaculture.2023.740166.
107 Stadtlander, T., F. Tschudi, A. Seitz, M. Sigrist, D. Refardt, and F. Leiber. 2023. Partial Replacement of Fishmeal with Duckweed (Spirodela polyrhiza) in Feed for Two Carnivorous Fish Species, Eurasian Perch (Perca fluviatilis) and Rainbow Trout (Oncorhynchus mykiss). Aquaculture Research 2023. https://doi.org/10.1155/2023/6680943.
108 Stejskal, Vlastimil, Hung Quang Tran, Marketa Prokesova, Tatyana Gebauer, Pham Thai Giang, Francesco Gai, and Laura Gasco. 2020. Partially Defatted Hermetia illucens Larva Meal in Diet of Eurasian Perch (Perca fluviatilis) Juveniles. Animals 10: 1876. https://doi.org/10.3390/ani10101876.
109 Tran, Hung Quang, Hien Van Doan, and Vlastimil Stejskal. 2021. Does dietary Tenebrio molitor affect swimming capacity, energy use, and physiological responses of European perch Perca fluviatilis? Aquaculture 539: 736610. https://doi.org/10.1016/j.aquaculture.2021.736610.
110 Tran, Hung Quang, Markéta Prokešová, Mahyar Zare, Jan Matoušek, Ilario Ferrocino, Laura Gasco, and Vlastimil Stejskal. 2022. Production performance, nutrient digestibility, serum biochemistry, fillet composition, intestinal microbiota and environmental impacts of European perch (Perca fluviatilis) fed defatted mealworm (Tenebrio molitor). Aquaculture 547: 737499. https://doi.org/10.1016/j.aquaculture.2021.737499.
111 Mood, Alison, Elena Lara, Natasha K. Boyland, and Phil Brooke. 2023. Estimating global numbers of farmed fishes killed for food annually from 1990 to 2019. Animal Welfare 32: e12. https://doi.org/10.1017/awf.2023.4.






Lorem ipsum
Something along the lines of: we were aware of the importance of some topics so that we wanted to include them and collect data but not score them. For WelfareChecks | farm, these topics are "domestication level", "feed replacement", and "commercial relevance". The domestication and commercial relevance aspects allow us to analyse the questions whether increasing rate of domestication or relevance in farming worldwide goes hand in hand with better welfare; the feed replacement rather goes in the direction of added suffering for all those species which end up as feed. For a carnivorous species, to gain 1 kg of meat, you do not just kill this one individual but you have to take into account the meat that it was fed during its life in the form of fish meal and fish oil. In other words, carnivorous species (and to a degree also omnivorous ones) have a larger "fish in:fish out" ratio.
Lorem ipsum
Probably, we updated the profile. Check the version number in the head of the page. For more information on the version, see the FAQ about this. Why do we update profiles? Not just do we want to include new research that has come out, but we are continuously developing the database itself. For example, we changed the structure of entries in criteria or we added explanations for scores in the WelfareCheck | farm. And we are always refining our scoring rules.
The centre of the Overview is an array of criteria covering basic features and behaviours of the species. Each of this information comes from our literature search on the species. If we researched a full Dossier on the species, probably all criteria in the Overview will be covered and thus filled. This was our way to go when we first set up the database.
Because Dossiers are time consuming to research, we switched to focusing on WelfareChecks. These are much shorter profiles covering just 10 criteria we deemed important when it comes to behaviour and welfare in aquaculture (and lately fisheries, too). Also, WelfareChecks contain the assessment of the welfare potential of a species which has become the main feature of the fair-fish database over time. Because WelfareChecks do not cover as many criteria as a Dossier, we don't have the information to fill all blanks in the Overview, as this information is "not investigated by us yet".
Our long-term goal is to go back to researching Dossiers for all species covered in the fair-fish database once we set up WelfareChecks for each of them. If you would like to support us financially with this, please get in touch at ffdb@fair-fish.net
See the question "What does "not investigated by us yet" mean?". In short, if we have not had a look in the literature - or in other words, if we have not investigated a criterion - we cannot know the data. If we have already checked the literature on a criterion and could not find anything, it is "no data found yet". You spotted a "no data found yet" where you know data exists? Get in touch with us at ffdb@fair-fish.net!
Once you have clicked on "show details", the entry for a criterion will unfold and display the summarised information we collected from the scientific literature – complete with the reference(s).
As reference style we chose "Springer Humanities (numeric, brackets)" which presents itself in the database as a number in a grey box. Mouse over the box to see the reference; click on it to jump to the bibliography at the bottom of the page. But what does "[x]-[y]" refer to?
This is the way we mark secondary citations. In this case, we read reference "y", but not reference "x", and cite "x" as mentioned in "y". We try to avoid citing secondary references as best as possible and instead read the original source ourselves. Sometimes we have to resort to citing secondarily, though, when the original source is: a) very old or not (digitally) available for other reasons, b) in a language no one in the team understands. Seldomly, it also happens that we are running out of time on a profile and cannot afford to read the original. As mentioned, though, we try to avoid it, as citing mistakes may always happen (and we don't want to copy the mistake) and as misunderstandings may occur by interpreting the secondarily cited information incorrectly.
If you spot a secondary reference and would like to send us the original work, please contact us at ffdb@fair-fish.net
In general, we aim at giving a good representation of the literature published on the respective species and read as much as we can. We do have a time budget on each profile, though. This is around 80-100 hours for a WelfareCheck and around 300 hours for a Dossier. It might thus be that we simply did not come around to reading the paper.
It is also possible, though, that we did have to make a decision between several papers on the same topic. If there are too many papers on one issue than we manage to read in time, we have to select a sample. On certain topics that currently attract a lot of attention, it might be beneficial to opt for the more recent papers; on other topics, especially in basic research on behaviour in the wild, the older papers might be the go-to source.
And speaking of time: the paper you are missing from the profile might have come out after the profile was published. For the publication date, please check the head of the profile at "cite this profile". We currently update profiles every 6-7 years.
If your paper slipped through the cracks and you would like us to consider it, please get in touch at ffdb@fair-fish.net
This number, for example "C | 2.1 (2022-11-02)", contains 4 parts:
- "C" marks the appearance – the design level – of the profile part. In WelfareChecks | farm, appearance "C" is our most recent one with consistent age class and label (WILD, FARM, LAB) structure across all criteria.
- "2." marks the number of major releases within this appearance. Here, it is major release 2. Major releases include e.g. changes of the WelfareScore. Even if we just add one paper – if it changes the score for one or several criteria, we will mark this as a major update for the profile. With a change to a new appearance, the major release will be re-set to 1.
- ".1" marks the number of minor updates within this appearance. Here, it is minor update 1. With minor updates, we mean changes in formatting, grammar, orthography. It can also mean adding new papers, but if these papers only confirm the score and don't change it, it will be "minor" in our book. With a change to a new appearance, the minor update will be re-set to 0.
- "(2022-11-02)" is the date of the last change – be it the initial release of the part, a minor, or a major update. The nature of the changes you may find out in the changelog next to the version number.
If an Advice, for example, has an initial release date and then just a minor update date due to link corrections, it means that – apart from correcting links – the Advice has not been updated in a major way since its initial release. Please take this into account when consulting any part of the database.
First up, you will find answers to questions for the specific page you are on. Scrolling down in the FAQ window, there are also answers to more general questions. Explore our website and the other sub pages and find there the answers to questions relevant for those pages.
In the fair-fish database, when you have chosen a species (either by searching in the search bar or in the species tree), the landing page is an Overview, introducing the most important information to know about the species that we have come across during our literatures search, including common names, images, distribution, habitat and growth characteristics, swimming aspects, reproduction, social behaviour but also handling details. To dive deeper, visit the Dossier where we collect all available ethological findings (and more) on the most important aspects during the life course, both biologically and concerning the habitat. In contrast to the Overview, we present the findings in more detail citing the scientific references.
Depending on whether the species is farmed or wild caught, you will be interested in different branches of the database.
Farm branch
Founded in 2013, the farm branch of the fair-fish database focuses on farmed aquatic species.
Catch branch
Founded in 2022, the catch branch of the fair-fish database focuses on wild-caught aquatic species.
The heart of the farm branch of the fair-fish database is the welfare assessment – or WelfareCheck | farm – resulting in the WelfareScore | farm for each species. The WelfareCheck | farm is a condensed assessment of the species' likelihood and potential for good welfare in aquaculture, based on welfare-related findings for 10 crucial criteria (home range, depth range, migration, reproduction, aggregation, aggression, substrate, stress, malformations, slaughter).
For those species with a Dossier, we conclude to-be-preferred farming conditions in the Advice | farm. They are not meant to be as detailed as a rearing manual but instead, challenge current farming standards and often take the form of what not to do.
In parallel to farm, the main element of the catch branch of the fair-fish database is the welfare assessment – or WelfareCheck | catch – with the WelfareScore | catch for each species caught with a specific catching method. The WelfareCheck | catch, too, is a condensed assessment of the species' likelihood and potential for good welfare – or better yet avoidance of decrease of good welfare – this time in fisheries. We base this on findings on welfare hazards in 10 steps along the catching process (prospection, setting, catching, emersion, release from gear, bycatch avoidance, sorting, discarding, storing, slaughter).
In contrast to the farm profiles, in the catch branch we assess the welfare separately for each method that the focus species is caught with. In the case of a species exclusively caught with one method, there will be one WelfareCheck, whereas in other species, there will be as many WelfareChecks as there are methods to catch the species with.
Summarising our findings of all WelfareChecks | catch for one species in Advice | catch, we conclude which catching method is the least welfare threatening for this species and which changes to the gear or the catching process will potentially result in improvements of welfare.
Welfare of aquatic species is at the heart of the fair-fish database. In our definition of welfare, we follow Broom (1986): “The welfare of an individual is its state as regards its attempts to cope with its environment.” Thus, welfare may be perceived as a continuum on which an individual rates “good” or “poor” or everything in between.
We pursue what could be called a combination of not only a) valuing the freedom from injuries and stress (function-based approach) but b) supporting attempts to provide rewarding experiences and cognitive challenges (feelings-based approach) as well as c) arguing for enclosures that mimic the wild habitat as best as possible and allow for natural behaviour (nature-based approach).
Try mousing over the element you are interested in - oftentimes you will find explanations this way. If not, there will be FAQ on many of the sub-pages with answers to questions that apply to the respective sub-page. If your question is not among those, contact us at ffdb@fair-fish.net.
It's right here! We decided to re-name it to fair-fish database for several reasons. The database has grown beyond dealing purely with ethology, more towards welfare in general – and so much more. Also, the partners fair-fish and FishEthoGroup decided to re-organise their partnership. While maintaining our friendship, we also desire for greater independence. So, the name "fair-fish database" establishes it as a fair-fish endeavour.