Information
Authors: Jenny Volstorf, Maria Filipa Castanheira
Version: C | 2.1Published: 2024-12-31
- minor editorial changes plus new side note "Commercial relevance"
WelfareScore | farm
The score card gives our welfare assessments for aquatic species in 10 criteria.
For each criterion, we score the probability to experience good welfare under minimal farming conditions ("Likelihood") and under high-standard farming conditions ("Potential") representing the worst and best case scenario. The third dimension scores how certain we are of our assessments based on the number and quality of sources we found ("Certainty").
The WelfareScore sums just the "High" scores in each dimension. Although good welfare ("High") seems not possible in some criteria, there could be at least a potential improvement from low to medium welfare (indicated by ➚ and the number of criteria).
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
➚ = potential improvements not reaching "High" - Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10 per dimension)
General remarks
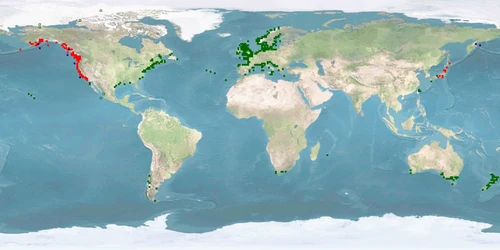
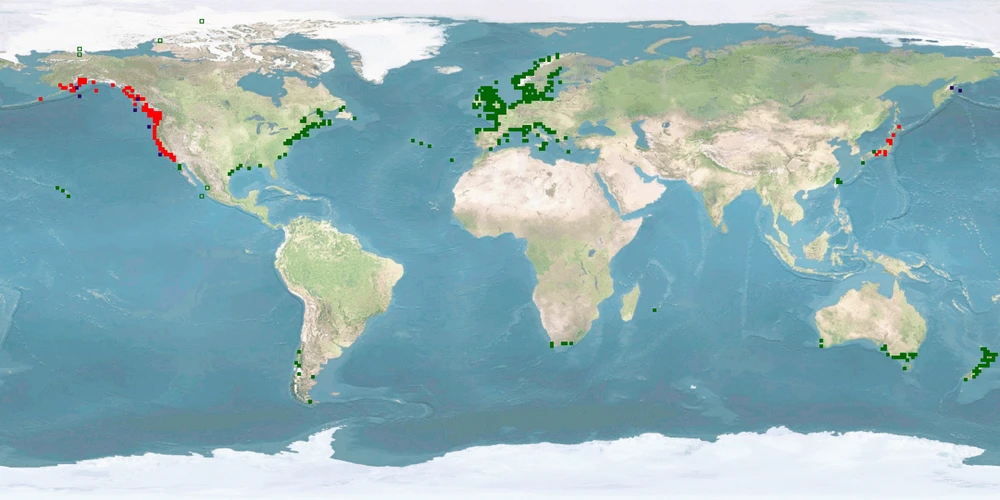
Oncorhynchus mykiss is one of the dominant salmonids farmed in Europe and North America, second only to Salmo salar. In addition, it is one of the most widely studied model fish species in the wild and in captivity. Yet, the living conditions and the husbandry systems that maximise the welfare of this species are still to be defined, developed, and improved. This lack is quite incomprehensible, given the background and the availability of research performed on this species.
The low WelfareScore is mainly due to the need of space and substrate and to high levels of aggression, stress, and high deformations under farming conditions. Grow-out typically takes place in ponds or raceways, sometimes also in cages. There are two strains in O. mykiss: the ANADROMOUS one, also called Steelhead trout, and the POTAMODROMOUS Rainbow trout. Aquaculture populations probably combine genes of both strains. In ANADROMOUS FISHES, throughout the life history, morphology, behaviour, and environmental requirements change. Husbandry systems and practices need to take such differences into account in order to achieve and maintain high welfare. The development of new rearing strategies to optimise the husbandry practices as well as handling with special care would be a step forward to solve some specific welfare concerns. Finally, providing feed which contains a lower amount of fish components from wild catch has proven feasible for this species in lab studies, so a protocol for application in farming conditions has to be developed.
Note: the name of the age classes differ in the two strains: LARVAE in Rainbow trout are called ALEVINS in Steelhead trout, JUVENILES in Rainbow trout are called PARR and SMOLTS in Steelhead trout, SPAWNERS in Rainbow trout are called GRILSE and KELTS in Steelhead trout. For consistency with other profiles, we will apply the usual age class structure of eggs, LARVAE, FRY, JUVENILES, ADULTS, SPAWNERS and make sure to signal the respective age class for Steelhead trout in the entries.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions, as the range in captivity at least overlaps with the range in the wild. Our conclusion is based on a medium amount of evidence, as wild information in larvae, fry, and at sea (Steelhead trout) as well as farm information in spawners is missing.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions, as the range in captivity at least overlaps with the range in the wild. Our conclusion is based on a medium amount of evidence.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal and high-standard farming conditions, as both strains undertake extensive migrations, and we cannot be sure that providing each age class with their respective environmental conditions will satisfy their urge to migrate or whether they need to experience the transition. Our conclusion is based on a medium amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of these circumstances?
It is low for minimal farming conditions. It is medium for high-standard farming conditions, as natural spawning is possible, and small farms simulate natural spawning conditions and do not apply hormonal manipulation, but omitting of separation of males and females (to be able to court) as well as omitting of stripping needs to be verified for the farming context. Our conclusion is based on a medium amount of evidence.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is low for minimal farming conditions, as – even in the absence of densities in the wild – we may conclude from laboratory studies that densities in raceways, cages, and some tanks are potentially stress inducing. It is medium for high-standard farming conditions, as lower stress at stocking densities in ponds and (some) tanks need to be verified for the farming context. Our conclusion is based on a medium amount of evidence.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal farming conditions, as aggression is present in all age classes, and size-grading does not seem to improve it. It is medium for high-standard farming conditions, as innovations to decrease aggression by adding enrichment need to be verified for the farming context. Our conclusion is based on a medium amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions, as the species uses substrate, but a) many or all farming facilities for each age class are devoid of it and b) given stripping in SPAWNERS. It is medium for high-standard farming conditions a) given earthen ponds which are not replaced by concrete or stone bottom and b) as innovations for enrichment need to be verified for the farming context. Our conclusion is based on a medium amount of evidence.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions. It is medium for high-standard farming conditions, as innovations to reduce stress need to be verified for the farming context. Our conclusions are based on a medium amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is low for minimal farming conditions, as malformation rates exceed 10%. It is medium for high-standard farming conditions, as some malformations result from conditions that may be changed (rearing intensity). Our conclusion is based on a low amount of evidence, as improvement of the situation by adjusting conditions needs more proof.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is low for minimal farming conditions. It is high for high-standard farming conditions, as a) percussive stunning followed by bleeding or b) electrical stunning followed by percussive killing or followed by bleeding induce unconsciousness fast, kill while still unconscious, and are verified for the farming context. Our conclusion is based on a high amount of evidence.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 5 85, fully domesticated. Cultured since late 19th century 4.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes:
- WILD: carnivorous (Rainbow trout) 86 87 20 (Rainbow and Steelhead trout) 63.
- FARM: no data found yet.
- LAB: FRY: fish meal in parallel to fish oil may be partly* replaced by sustainable sources 88. JUVENILES: fish meal may be mostly* 89, fish oil party* replaced by sustainable sources 90. Fish meal in parallel to fish oil may be partly* replaced by sustainable sources 88 or completely* if it had already been given for first feeding 7 months prior 91. ADULTS: fish meal may be mostly* replaced by sustainable sources 92.
*partly = <51% – mostly = 51-99% – completely = 100%
Side note: Commercial relevance
How much is this species farmed annually?
916,540 t/year 1990-2019 amounting to estimated 1,022,000,000-1,809,000,000 IND/year 1990-2019 93.
Glossary
ALEVINS = larvae until the end of yolk sac absorption
ANADROMOUS = migrating from the sea into fresh water to spawn
DOMESTICATION LEVEL 5 = selective breeding programmes are used focusing on specific goals 85
EURYHALINE = tolerant of a wide range of salinities
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FISHES = using "fishes" instead of "fish" for more than one individual - whether of the same species or not - is inspired by Jonathan Balcombe who proposed this usage in his book "What a fish knows". By referring to a group as "fishes", we acknowledge the individuals with their personalities and needs instead of an anonymous mass of "fish".
FRY = larvae from external feeding on
GRILSE = adults returning from sea to home river to spawn
IND = individuals
JUVENILES = fully developed but immature individuals
KELTS = adults surviving spawning
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening
PARR = juvenile stage in rivers
PHOTOPERIOD = duration of daylight
POTAMODROMOUS = migrating within fresh water
SMOLTS = juvenile stage migrating to the sea
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
WILD = setting in the wild
Bibliography
2 Gustafson-Greenwood, Karla I., and John R. Moring. 1990. Territory size and distribution of newly-emerged Atlantic salmon (Salmo salar). Hydrobiologia 206: 125–131. https://doi.org/10.1007/BF00018638.
3 Okumuş, İbrahim. 2002. Rainbow Trout Broodstock Management and Seed Production in Turkey: Present Practices, Constraints and the Future. Turkish Journal of Fisheries and Aquatic Sciences 2: 16.
4 Cowx, I. G. 2005. Cultured Aquatic Species Information Programme. Oncorhynchus mykiss. Rome: FAO Fisheries and Aquaculture Department.
5 Woynarovich, András, György Hoitsy, and Thomas Moth-Poulsen. 2011. Small-scale rainbow trout farming. FAO Fisheries and Aquaculture Technical Paper 561. Rome: Food and Agriculture Organization of the United Nations.
6 Erman, Don C., and George R. Leidy. 1975. Downstream Movement of Rainbow Trout Fry in a Tributary Sagehen Creek, under Permanent and Intermittent Flow. Transactions of the American Fisheries Society 104: 467–473. https://doi.org/10.1577/1548-8659(1975)104<467:DMORTF>2.0.CO;2.
7 Mitro, Matthew G., and Alexander V. Zale. 2002. Seasonal Survival, Movement, and Habitat Use of Age-0 Rainbow Trout in the Henrys Fork of the Snake River, Idaho. Transactions of the American Fisheries Society 131: 271–286. https://doi.org/10.1577/1548-8659(2002)131<0271:SSMAHU>2.0.CO;2.
8 Mellina, Eric, Scott G. Hinch, Kirsten D. MacKenzie, and Greg Pearson. 2005. Seasonal Movement Patterns of Stream-Dwelling Rainbow Trout in North-Central British Columbia, Canada. Transactions of the American Fisheries Society 134: 1021–1037. https://doi.org/10.1577/T03-188.1.
9 Cocherell, Sarah A., Gardner J. Jones, Javier B. Miranda, Dennis E. Cocherell, Joseph J. Cech, Lisa C. Thompson, and A. Peter Klimley. 2010. Distribution and movement of domestic rainbow trout, Oncorhynchus mykiss, during pulsed flows in the South Fork American River, California. Environmental Biology of Fishes 89: 105–116. https://doi.org/10.1007/s10641-010-9701-2.
10 Pulcini, D., C. Boglione, E. Palamara, and S. Cataudella. 2010. Use of meristic counts and skeletal anomalies to assess developmental plasticity of farmed rainbow trout (Oncorhynchus mykiss, Walbaum 1792): a preliminary study. Journal of Applied Ichthyology 26: 298–302. https://doi.org/10.1111/j.1439-0426.2010.01425.x.
11 Boglione, Clara, Domitilla Pulcini, Michele Scardi, Elisa Palamara, Tommaso Russo, and Stefano Cataudella. 2014. Skeletal Anomaly Monitoring in Rainbow Trout (Oncorhynchus mykiss , Walbaum 1792) Reared under Different Conditions. PLOS ONE 9: e96983. https://doi.org/10.1371/journal.pone.0096983.
12 Bolzonella, Matteo, Edouard Royer, Adriano C. Lima, and Roberto Pastres. 2022. Predicting farmed rainbow trout weight distribution to improve feeding practice: an individual-based model approach. Open Research Europe.
13 Noble, C., K. Gismervik, M.H. Iversen, J. Kolarevic, J. Nilsson, L.H. Stien, and J. F. Turnbull, ed. 2020. Welfare Indicators for farmed rainbow trout: tools for assessing fish welfare.
14 Gido, Keith B., Robert D. Larson, and Lief A. Ahlm. 2000. Stream-Channel Position of Adult Rainbow Trout Downstream of Navajo Reservoir, New Mexico, Following Changes inReservoir Release. North American Journal of Fisheries Management 20: 250–258. https://doi.org/10.1577/1548-8675(2000)020<0250:SCPOAR>2.0.CO;2.
15 Meka, J, E. Knudsen, D. Douglas, and R. Benter. 2003. Variable migratory patterns of different adult rainbow trout life history types in a southwest Alaska watershed. Journal Articles 132.
16 James, G. D., and J. R. M. Kelso. 1995. Movements and habitat preference of adult rainbow trout (Oncorhynchus mykiss) in a New Zealand montane lake. New Zealand Journal of Marine and Freshwater Research 29: 493–503. https://doi.org/10.1080/00288330.1995.9516682.
17 Venman, Mark R., and Michel Dedual. 2005. Migratory behaviour of spawning rainbow trout (Oncorhynchus mykiss) in the Tongariro River, New Zealand, after habitat alteration. New Zealand Journal of Marine and Freshwater Research 39: 951–961. https://doi.org/10.1080/00288330.2005.9517365.
18 Shapovalov, Leo, and Alan C. Taft. 1954. The Life Histories of the Steelhead Rainbow Trout (Salmo gairdneri gairdneri) and Silver Salmon (Oncorhynchus kisutch) With Special Reference to Waddell Creek, California, and Recommendations Regarding Their Management. Fish Bulletin 98. State of California Department of Fish and Game.
19 Hoitsy, György, András Woynarovich, and Thomas Moth-Poulsen. 2012. Guide to the small scale artificial propagation of trout. Budapest: Food and Agriculture Organization of the United Nations.
20 Riehle, Michael D., and J. S. Griffith. 1993. Changes in Habitat Use and Feeding Chronology of Juvenile Rainbow Trout (Oncorhynchus mykiss) in Fall and the Onset of Winter in Silver Creek, Idaho. Canadian Journal of Fisheries and Aquatic Sciences 50: 2119–2128. https://doi.org/10.1139/f93-237.
21 Vondracek, B., and D. R. Longanecker. 1993. Habitat selection by rainbow trout Oncorhynchus mykiss in a California stream: implications for the Instream Flow Incremental Methodology. Ecology of Freshwater Fish 2: 173–186. https://doi.org/10.1111/j.1600-0633.1993.tb00100.x.
22 Matthews, K. R., and N. H. Berg. 1997. Rainbow trout responses to water temperature and dissolved oxygen stress in two southern California stream pools. Journal of Fish Biology 50: 50–67. https://doi.org/10.1111/j.1095-8649.1997.tb01339.x.
23 Ebersole, J. L., W. J. Liss, and C. A. Frissell. 2001. Relationship between stream temperature, thermal refugia and rainbow trout Oncorhynchus mykiss abundance in arid-land streams in the northwestern United States. Ecology of Freshwater Fish 10: 1–10. https://doi.org/10.1034/j.1600-0633.2001.100101.x.
24 Ruggerone, G., and T. P. Quinn. 1989. Unpublished data.
25 Light, Jeffrey T, Cohn K Harris, and Robert L Burgner. 1989. Ocean distribution and migration of steelhead (Oncorhynchus mykiss, formerly Salmo gairdneri). Document submitted to the International North Pacific Fisheries Commission. FRI-UW-8912. Seattle: FisheriesResearch Institute, University of Washington.
26 Jokumsen, Alfred, and Lars M Svendsen. 2010. Farming of Freshwater Rainbow Trout in Denmark. DTU-Aqua Rapport 219: 51.
27 Cardia, Francesco, and Alessandro Lovatelli. 2015. Aquaculture operations in floating HDPE cages: a field handbook. FAO Fisheries and Aquaculture Technical Paper 593. Rome: Food and Agriculture Organization of the United Nations.
28 Riva Rossi, Carla, Milagros Arguimbau, and Miguel Pascual. 2003. The spawning migration of anadromous rainbow trout in the Santa Cruz River, Patagonia (Argentina) through radio-tracking. Ecología austral 13: 151–159.
29 Hayes, Sean A., Morgan H. Bond, Chad V. Hanson, Andrew W. Jones, Arnold J. Ammann, Jeffrey A. Harding, Alison L. Collins, Jeffrey Perez, and R. Bruce MacFarlane. 2011. Down, up, down and “smolting” twice? Seasonal movement patterns by juvenile steelhead (Oncorhynchus mykiss) in a coastal watershed with a bar closing estuary. Canadian Journal of Fisheries and Aquatic Sciences 68: 1341–1350. https://doi.org/10.1139/f2011-062.
30 Bradford, Michael J, and Paul S Higgins. 2001. Habitat-, season-, and size-specific variation in diel activity patterns of juvenile chinook salmon (Oncorhynchus tshawytscha) and steelhead trout (Oncorhynchus mykiss). Canadian Journal of Fisheries and Aquatic Sciences 58: 365–374. https://doi.org/10.1139/f00-253.
31 Uiuiu, Paul, Călin Lațiu, Tudor Păpuc, Cristina Craioveanu, Andrada Ihuț, Alexandru Sava, Camelia Răducu, et al. 2021. Multi-Approach Assessment for Stress Evaluation in Rainbow Trout Females, Oncorhynchus mykiss (Walbaum, 1792) from Three Different Farms during the Summer Season. Animals 11: 1810. https://doi.org/10.3390/ani11061810.
32 Albrektsen, S., and O. Torrissen. 1988. Physiological changes in blood and seminal plasma during the spawning period of maturing rainbow trout held under different temperature and salinity regimes, and the effect on survival of the broodstock and the eyed eggs. Nofima.
33 Froese, R., and D. Pauly. 2020. Oncorhynchus mykiss, Rainbow trout: fisheries, aquaculture, gamefish. www.fishbase.org. https://www.fishbase.de/summary/Oncorhynchus-mykiss.html. Accessed August 12.
34 Needham, P. R., and Alan C. Taft. 1934. Observations on the spawning of steelhead trout. Transactions of the American Fisheries Society 64: 332–338.
35 Tautz, A. F., and C. Groot. 1975. Spawning Behavior of Chum Salmon (Oncorhynchus keta) and Rainbow Trout (Salmo gairdneri). Journal of the Fisheries Research Board of Canada 32: 633–642. https://doi.org/10.1139/f75-081.
36 Bonnet, Emilie, Alexis Fostier, and Julien Bobe. 2007. Characterization of rainbow trout egg quality: A case study using four different breeding protocols, with emphasis on the incidence of embryonic malformations. Theriogenology 67: 786–794. https://doi.org/10.1016/j.theriogenology.2006.10.008.
37 Hoitsy, György. 2002. A Pisztráng tenyésztése és horgászata.
38 Weirup, Lina, Carsten Schulz, Henrike Seibel, and Johan Aerts. 2021. Scale cortisol is positively correlated to fin injuries in rainbow trout (Oncorhynchus mykiss) reared in commercial flow through systems. Aquaculture 543: 736924. https://doi.org/10.1016/j.aquaculture.2021.736924.
39 Bégout Anras, Marie-Laure, and Jean Paul Lagardère. 2004. Measuring cultured fish swimming behaviour: first results on rainbow trout using acoustic telemetry in tanks. Aquaculture 240: 175–186. https://doi.org/10.1016/j.aquaculture.2004.02.019.
40 Pickering, A. D., T. G. Pottinger, J. P. Sumpter, J. F. Carragher, and P. Y. Le Bail. 1991. Effects of acute and chronic stress on the levels of circulating growth hormone in the rainbow trout, Oncorhynchus mykiss. General and Comparative Endocrinology 83: 86–93. https://doi.org/10.1016/0016-6480(91)90108-I.
41 North, B. P., J. F. Turnbull, T. Ellis, M. J. Porter, H. Migaud, J. Bron, and N. R. Bromage. 2006. The impact of stocking density on the welfare of rainbow trout (Oncorhynchus mykiss). Aquaculture 255: 466–479. https://doi.org/10.1016/j.aquaculture.2006.01.004.
42 Brunet, Valentin, Aude Kleiber, Amélie Patinote, Pierre-Lô Sudan, Cécile Duret, Guillaume Gourmelen, Emmanuelle Moreau, et al. 2022. Positive welfare effects of physical enrichments from the nature-, functions- and feeling- based approaches in farmed rainbow trout (Oncorhynchus mykiss). Aquaculture 550: 737825. https://doi.org/10.1016/j.aquaculture.2021.737825.
43 Fried, Stephen M., James D. McCleave, and George W. LaBar. 1978. Seaward Migration of Hatchery-Reared Atlantic Salmon, Salmo salar, Smolts in the Penobscot River Estuary, Maine: Riverine Movements. Journal of the Fisheries Research Board of Canada 35: 76–87. https://doi.org/10.1139/f78-011.
44 McCormick, Stephen D, Lars P Hansen, Thomas P Quinn, and Richard L Saunders. 1998. Movement, migration, and smolting of Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 55: 77–92. https://doi.org/10.1139/d98-011.
45 Riley, W. D., A. T. Ibbotson, D. L. Maxwell, P. I. Davison, W. R. C. Beaumont, and M. J. Ives. 2014. Development of schooling behaviour during the downstream migration of Atlantic salmon Salmo salar smolts in a chalk stream. Journal of Fish Biology 85: 1042–1059. https://doi.org/10.1111/jfb.12457.
46 Tatara, Christopher P, Stephen C Riley, and Julie A Scheurer. 2008. Environmental enrichment in steelhead (Oncorhynchus mykiss) hatcheries: field evaluation of aggression, foraging, and territoriality in natural and hatchery fry. Canadian Journal of Fisheries and Aquatic Sciences 65: 744–753. https://doi.org/10.1139/f08-004.
47 Berejikian, Barry A, E Paul Tezak, Thomas A Flagg, Anita L LaRae, Eric Kummerow, and Conrad VW Mahnken. 2000. Social dominance, growth, and habitat use of age-0 steelhead (Oncorhynchus mykiss) grown in enriched and conventional hatchery rearing environments. Canadian Journal of Fisheries and Aquatic Sciences 57: 628–636. https://doi.org/10.1139/f99-288.
48 Klíma, Ondřej, Lukáš Kohút, Jan Mareš, and Radovan Kopp. 2018. The Effect of Feeding Frequency on the Fin Condition in Rainbow Trout (Oncorhynchus mykiss). Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis 66: 669–675. https://doi.org/10.11118/actaun201866030669.
49 Noble, C., K. Mizusawa, K. Suzuki, and M. Tabata. 2007. The effect of differing self-feeding regimes on the growth, behaviour and fin damage of rainbow trout held in groups. Aquaculture 264: 214–222. https://doi.org/10.1016/j.aquaculture.2006.12.028.
50 Øverli, Øyvind, Charmaine A. Harris, and Svante Winberg. 1999. Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain, Behavior and Evolution 54: 263–275. https://doi.org/10.1159/000006627.
51 Basic, D., S. Winberg, J. Schjolden, Å. Krogdahl, and E. Höglund. 2012. Context-dependent responses to novelty in Rainbow trout (Oncorhynchus mykiss), selected for high and low post-stress cortisol responsiveness. Physiology & Behavior 105: 1175–1181. https://doi.org/10.1016/j.physbeh.2011.12.021.
52 Toobaie, Asra, and James W. A. Grant. 2013. Effect of food abundance on aggressiveness and territory size of juvenile rainbow trout, Oncorhynchus mykiss. Animal Behaviour 85: 241–246. https://doi.org/10.1016/j.anbehav.2012.10.032.
53 Moutou, K. A., I. D. McCarthy, and D. F. Houlihan. 1998. The effect of ration level and social rank on the development of fin damage in juvenile rainbow trout. Journal of Fish Biology 52: 756–770. https://doi.org/10.1111/j.1095-8649.1998.tb00818.x.
54 Gregory, T Ryan, and Chris M Wood. 1999. Interactions between individual feeding behaviour, growth, and swimming performance in juvenile rainbow trout (Oncorhynchus mykiss) fed different rations. Canadian Journal of Fisheries and Aquatic Sciences 56: 479–486. https://doi.org/10.1139/f98-186.
55 Kleiber, Aude, Jean-Michel Le-Calvez, Thierry Kerneis, Axel Batard, Lionel Goardon, Laurent Labbé, Valentin Brunet, et al. 2022. Positive effects of bubbles as a feeding predictor on behaviour of farmed rainbow trout. Scientific Reports 12: 11368. https://doi.org/10.1038/s41598-022-15302-7.
56 Sowden, Terry K., and G. Power. 1985. Prediction of Rainbow Trout Embryo Survival in Relation to Groundwater Seepage and Particle Size of Spawning Substrates. Transactions of the American Fisheries Society 114: 804–812. https://doi.org/10.1577/1548-8659(1985)114<804:PORTES>2.0.CO;2.
57 Workman, R. D., Daniel B. Hayes, and Thomas G. Coon. 2004. Spawning Habitat Selection by Rainbow Trout in the Pere Marquette River, Michigan. Journal of Great Lakes Research 30: 397–406. https://doi.org/10.1016/S0380-1330(04)70357-3.
58 Bosakowski, Thomas, and Eric J. Wagner. 1995. Experimental use of cobble substrates in concrete raceways for improving fin condition of cutthroat (Oncorhynchus clarki) and rainbow trout (O. mykiss). Aquaculture 130: 159–165. https://doi.org/10.1016/0044-8486(94)00223-B.
59 Becket, Kristen H, and Michael Barnes. 2015. Rearing with overhead cover influences rainbow trout behavior. Proceedings of the South Dakota Academy of Science 94: 187–193.
60 Arndt, Ronney E., M. Douglas Routledge, Eric J. Wagner, and Roger F. Mellenthin. 2001. Influence of Raceway Substrate and Design on Fin Erosion and Hatchery Performance of Rainbow Trout. North American Journal of Aquaculture 63: 312–320. https://doi.org/10.1577/1548-8454(2001)063<0312:IORSAD>2.0.CO;2.
61 Zydlewski, Gayle B., J. Scott Foott, Kenneth Nichols, Scott Hamelberg, Joseph Zydlewski, and Björn Thrandur Björnsson. 2003. Enhanced smolt characteristics of steelhead trout exposed to alternative hatchery conditions during the final months of rearing. Aquaculture 222. Salmonid Smoltification: 101–117. https://doi.org/10.1016/S0044-8486(03)00105-4.
62 Tipping, Jack M. 2008. Adult Returns of Hatchery Steelhead Juveniles Reared in Earthen- and Asphalt-Bottom Ponds. North American Journal of Aquaculture 70: 115–117. https://doi.org/10.1577/A07-017.1.
63 McMichael, Geoffrey A., Cameron S. Sharpe, and Todd N. Pearsons. 1997. Effects of Residual Hatchery-Reared Steelhead on Growth of Wild Rainbow Trout and Spring Chinook Salmon. Transactions of the American Fisheries Society 126: 230–239. https://doi.org/10.1577/1548-8659(1997)126<0230:EORHRS>2.3.CO;2.
64 Villarroel, Morris, Genaro C. Miranda-de la Lama, Rubén Bermejo-Poza, Concepción Pérez, Elisabet González-de Chávarri, Fernando Torrent, and Jesús De la Fuente. 2021. Effects of Randomly Fired Underwater Currents as an Occupational Enrichment Program in Rainbow Trout (Oncorhynchus mykiss). Water 13: 3057. https://doi.org/10.3390/w13213057.
65 Meyer, K. A., and J. S. Gregory. 2000. Evidence of concealment behavior by adult rainbow trout and brook trout in winter. Ecology of Freshwater Fish 9: 138–144. https://doi.org/10.1111/j.1600-0633.2000.eff090302.x.
66 Barry, T. P., J. A. Malison, J. A. Held, and J. J. Parrish. 1995. Ontogeny of the cortisol stress response in larval rainbow trout. General and Comparative Endocrinology 97: 57–65. https://doi.org/10.1006/gcen.1995.1006.
67 EFSA. 2009. Species-specific welfare aspects of the main systems of stunning and killing of farmed fish: Rainbow Trout. EFSA Journal 7: 1012. https://doi.org/10.2903/j.efsa.2009.1012.
68 Tacchi, Luca, Liam Lowrey, Rami Musharrafieh, Kyle Crossey, Erin T. Larragoite, and Irene Salinas. 2015. Effects of transportation stress and addition of salt to transport water on the skin mucosal homeostasis of rainbow trout (Oncorhynchus mykiss). Aquaculture 435: 120–127. https://doi.org/10.1016/j.aquaculture.2014.09.027.
69 Shabani, Fazli, Ulf Erikson, Elvira Beli, and Agim Rexhepi. 2016. Live transport of rainbow trout (Onchorhynchus mykiss) and subsequent live storage in market: Water quality, stress and welfare considerations. Aquaculture 453: 110–115. https://doi.org/10.1016/j.aquaculture.2015.11.040.
70 Sneddon, Lynne U, Victoria A Braithwaite, and Michael J Gentle. 2003. Novel object test: examining nociception and fear in the rainbow trout. The Journal of Pain 4: 431–440. https://doi.org/10.1067/S1526-5900(03)00717-X.
71 Ellis, T., J. D. James, C. Stewart, and A. P. Scott. 2004. A non-invasive stress assay based upon measurement of free cortisol released into the water by rainbow trout. Journal of Fish Biology 65: 1233–1252. https://doi.org/10.1111/j.0022-1112.2004.00499.x.
72 Jentoft, Sissel, Are H. Aastveit, Peter A. Torjesen, and Øivind Andersen. 2005. Effects of stress on growth, cortisol and glucose levels in non-domesticated Eurasian perch (Perca fluviatilis) and domesticated rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 141: 353–358. https://doi.org/10.1016/j.cbpb.2005.06.006.
73 Kubi̇lay, Ayşegül, and Gülşen Uluköy. 2002. The effects of acute stress on rainbow trout (Oncorhynchus mykiss). Turkish Journal of Zoology 26: 249–254.
74 Karakatsouli, Nafsika, Sofronios E. Papoutsoglou, Georgios Panopoulos, Eustratios S. Papoutsoglou, Stella Chadio, and Dimitris Kalogiannis. 2008. Effects of light spectrum on growth and stress response of rainbow trout Oncorhynchus mykiss reared under recirculating system conditions. Aquacultural Engineering 38: 36–42. https://doi.org/10.1016/j.aquaeng.2007.10.006.
75 Vijayan, M. M., and T. W. Moon. 1992. Acute Handling Stress Alters Hepatic Glycogen Metabolism in Food-Deprived Rainbow Trout (Oncorhynchus mykiss). Canadian Journal of Fisheries and Aquatic Sciences 49: 2260–2266. https://doi.org/10.1139/f92-247.
76 Hoskonen, Petri, and Juhani Pirhonen. 2006. Effects of repeated handling, with or without anaesthesia, on feed intake and growth in juvenile rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture Research 37: 409–415. https://doi.org/10.1111/j.1365-2109.2005.01448.x.
77 Wagner, Eric, Ronney Arndt, and Blaine Hilton. 2002. Physiological stress responses, egg survival and sperm motility for rainbow trout broodstock anesthetized with clove oil, tricaine methanesulfonate or carbon dioxide. Aquaculture 211: 353–366. https://doi.org/10.1016/S0044-8486(01)00878-X.
78 Deschamps, M. -H., A. Kacem, R. Ventura, G. Courty, P. Haffray, F. J. Meunier, and J. -Y. Sire. 2008. Assessment of “discreet” vertebral abnormalities, bone mineralization and bone compactness in farmed rainbow trout. Aquaculture 279: 11–17. https://doi.org/10.1016/j.aquaculture.2008.03.036.
79 D’Agaro, Edo, PierPaolo Gibertoni, and Stefano Esposito. 2022. Recent Trends and Economic Aspects in the Rainbow Trout (Oncorhynchus mykiss) Sector. Applied Sciences 12: 8773. https://doi.org/10.3390/app12178773.
80 Lines, J. A., D. H. Robb, S. C. Kestin, S. C. Crook, and T. Benson. 2003. Electric stunning: a humane slaughter method for trout. Aquacultural Engineering 28: 141–154. https://doi.org/10.1016/S0144-8609(03)00021-9.
81 Jung-Schroers, Verena, Uta Hildebrandt, Karina Retter, Karl-Heinz Esser, John Hellmann, Dirk Willem Kleingeld, Karl Rohn, and Dieter Steinhagen. 2020. Is humane slaughtering of rainbow trout achieved in conventional production chains in Germany? Results of a pilot field and laboratory study. BMC Veterinary Research 16. https://doi.org/10.1186/s12917-020-02412-5.
82 Kestin, S. C., S. B. Wotton, and N. G. Gregory. 1991. Effect of slaughter by removal from water on visual evoked activity in the brain and reflex movement of rainbow trout (Oncorhynchus mykiss). The Veterinary Record 128: 443–446.
83 Robb, D. H. F, M O’ Callaghan, J. A Lines, and S. C Kestin. 2002. Electrical stunning of rainbow trout (Oncorhynchus mykiss): factors that affect stun duration. Aquaculture 205: 359–371. https://doi.org/10.1016/S0044-8486(01)00677-9.
84 Concollato, Anna, Rolf Erik Olsen, Sheyla Cristina Vargas, Antonio Bonelli, Marco Cullere, and Giuliana Parisi. 2016. Effects of stunning/slaughtering methods in rainbow trout (Oncorhynchus mykiss) from death until rigor mortis resolution. Aquaculture 464: 74–79. https://doi.org/10.1016/j.aquaculture.2016.06.009.
85 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
86 Rowe, D. K. 1984. Factors affecting the foods and feeding patterns of lake‐dwelling rainbow trout (Salmo gairdnerii) in the North Island of New Zealand. New Zealand Journal of Marine and Freshwater Research 18: 129–141. https://doi.org/10.1080/00288330.1984.9516036.
87 Kusabs, Ian A., and Stephen Swales. 1991. Diet and food resource partitioning in koaro, Galaxias brevipinnis (Günther), and juvenile rainbow trout, Oncorhynchus mykiss (Richardson), in two Taupo streams, New Zealand. New Zealand Journal of Marine and Freshwater Research 25: 317–325. https://doi.org/10.1080/00288330.1991.9516485.
88 Lazzarotto, Viviana, Françoise Médale, Laurence Larroquet, and Geneviève Corraze. 2018. Long-term dietary replacement of fishmeal and fish oil in diets for rainbow trout (Oncorhynchus mykiss): Effects on growth, whole body fatty acids and intestinal and hepatic gene expression. PLOS ONE 13: e0190730. https://doi.org/10.1371/journal.pone.0190730.
89 Gomes, Emídio F., Paulo Rema, and Sadasivam J. Kaushik. 1995. Replacement of fish meal by plant proteins in the diet of rainbow trout (Oncorhynchus mykiss): digestibility and growth performance. Aquaculture 130: 177–186. https://doi.org/10.1016/0044-8486(94)00211-6.
90 Örnek, Eda, Ümit Acar, and Mustafa Öğütcü. 2021. Effects of dietary fish oil replacement by poppy seed oil on growth performance and fillet quality of rainbow trout (Oncorhynchus mykiss). Aquaculture Research 52: 3026–3037. https://doi.org/10.1111/are.15147.
91 Geurden, Inge, Peter Borchert, Mukundh N. Balasubramanian, Johan W. Schrama, Mathilde Dupont-Nivet, Edwige Quillet, Sadasivam J. Kaushik, Stéphane Panserat, and Françoise Médale. 2013. The Positive Impact of the Early-Feeding of a Plant-Based Diet on Its Future Acceptance and Utilisation in Rainbow Trout. PLOS ONE 8: e83162. https://doi.org/10.1371/journal.pone.0083162.
92 Voorhees, Jill M., Michael E. Barnes, Steven R. Chipps, and Michael L. Brown. 2019. Bioprocessed soybean meal replacement of fish meal in rainbow trout (Oncorhynchus mykiss) diets. Edited by Pedro González-Redondo. Cogent Food & Agriculture 5: 1579482. https://doi.org/10.1080/23311932.2019.1579482.
93 Mood, Alison, Elena Lara, Natasha K. Boyland, and Phil Brooke. 2023. Estimating global numbers of farmed fishes killed for food annually from 1990 to 2019. Animal Welfare 32: e12. https://doi.org/10.1017/awf.2023.4.










Lorem ipsum
Something along the lines of: we were aware of the importance of some topics so that we wanted to include them and collect data but not score them. For WelfareChecks | farm, these topics are "domestication level", "feed replacement", and "commercial relevance". The domestication and commercial relevance aspects allow us to analyse the questions whether increasing rate of domestication or relevance in farming worldwide goes hand in hand with better welfare; the feed replacement rather goes in the direction of added suffering for all those species which end up as feed. For a carnivorous species, to gain 1 kg of meat, you do not just kill this one individual but you have to take into account the meat that it was fed during its life in the form of fish meal and fish oil. In other words, carnivorous species (and to a degree also omnivorous ones) have a larger "fish in:fish out" ratio.
Lorem ipsum
Probably, we updated the profile. Check the version number in the head of the page. For more information on the version, see the FAQ about this. Why do we update profiles? Not just do we want to include new research that has come out, but we are continuously developing the database itself. For example, we changed the structure of entries in criteria or we added explanations for scores in the WelfareCheck | farm. And we are always refining our scoring rules.
The centre of the Overview is an array of criteria covering basic features and behaviours of the species. Each of this information comes from our literature search on the species. If we researched a full Dossier on the species, probably all criteria in the Overview will be covered and thus filled. This was our way to go when we first set up the database.
Because Dossiers are time consuming to research, we switched to focusing on WelfareChecks. These are much shorter profiles covering just 10 criteria we deemed important when it comes to behaviour and welfare in aquaculture (and lately fisheries, too). Also, WelfareChecks contain the assessment of the welfare potential of a species which has become the main feature of the fair-fish database over time. Because WelfareChecks do not cover as many criteria as a Dossier, we don't have the information to fill all blanks in the Overview, as this information is "not investigated by us yet".
Our long-term goal is to go back to researching Dossiers for all species covered in the fair-fish database once we set up WelfareChecks for each of them. If you would like to support us financially with this, please get in touch at ffdb@fair-fish.net
See the question "What does "not investigated by us yet" mean?". In short, if we have not had a look in the literature - or in other words, if we have not investigated a criterion - we cannot know the data. If we have already checked the literature on a criterion and could not find anything, it is "no data found yet". You spotted a "no data found yet" where you know data exists? Get in touch with us at ffdb@fair-fish.net!
Once you have clicked on "show details", the entry for a criterion will unfold and display the summarised information we collected from the scientific literature – complete with the reference(s).
As reference style we chose "Springer Humanities (numeric, brackets)" which presents itself in the database as a number in a grey box. Mouse over the box to see the reference; click on it to jump to the bibliography at the bottom of the page. But what does "[x]-[y]" refer to?
This is the way we mark secondary citations. In this case, we read reference "y", but not reference "x", and cite "x" as mentioned in "y". We try to avoid citing secondary references as best as possible and instead read the original source ourselves. Sometimes we have to resort to citing secondarily, though, when the original source is: a) very old or not (digitally) available for other reasons, b) in a language no one in the team understands. Seldomly, it also happens that we are running out of time on a profile and cannot afford to read the original. As mentioned, though, we try to avoid it, as citing mistakes may always happen (and we don't want to copy the mistake) and as misunderstandings may occur by interpreting the secondarily cited information incorrectly.
If you spot a secondary reference and would like to send us the original work, please contact us at ffdb@fair-fish.net
In general, we aim at giving a good representation of the literature published on the respective species and read as much as we can. We do have a time budget on each profile, though. This is around 80-100 hours for a WelfareCheck and around 300 hours for a Dossier. It might thus be that we simply did not come around to reading the paper.
It is also possible, though, that we did have to make a decision between several papers on the same topic. If there are too many papers on one issue than we manage to read in time, we have to select a sample. On certain topics that currently attract a lot of attention, it might be beneficial to opt for the more recent papers; on other topics, especially in basic research on behaviour in the wild, the older papers might be the go-to source.
And speaking of time: the paper you are missing from the profile might have come out after the profile was published. For the publication date, please check the head of the profile at "cite this profile". We currently update profiles every 6-7 years.
If your paper slipped through the cracks and you would like us to consider it, please get in touch at ffdb@fair-fish.net
This number, for example "C | 2.1 (2022-11-02)", contains 4 parts:
- "C" marks the appearance – the design level – of the profile part. In WelfareChecks | farm, appearance "C" is our most recent one with consistent age class and label (WILD, FARM, LAB) structure across all criteria.
- "2." marks the number of major releases within this appearance. Here, it is major release 2. Major releases include e.g. changes of the WelfareScore. Even if we just add one paper – if it changes the score for one or several criteria, we will mark this as a major update for the profile. With a change to a new appearance, the major release will be re-set to 1.
- ".1" marks the number of minor updates within this appearance. Here, it is minor update 1. With minor updates, we mean changes in formatting, grammar, orthography. It can also mean adding new papers, but if these papers only confirm the score and don't change it, it will be "minor" in our book. With a change to a new appearance, the minor update will be re-set to 0.
- "(2022-11-02)" is the date of the last change – be it the initial release of the part, a minor, or a major update. The nature of the changes you may find out in the changelog next to the version number.
If an Advice, for example, has an initial release date and then just a minor update date due to link corrections, it means that – apart from correcting links – the Advice has not been updated in a major way since its initial release. Please take this into account when consulting any part of the database.
Lorem ipsum
In the fair-fish database, when you have chosen a species (either by searching in the search bar or in the species tree), the landing page is an Overview, introducing the most important information to know about the species that we have come across during our literatures search, including common names, images, distribution, habitat and growth characteristics, swimming aspects, reproduction, social behaviour but also handling details. To dive deeper, visit the Dossier where we collect all available ethological findings (and more) on the most important aspects during the life course, both biologically and concerning the habitat. In contrast to the Overview, we present the findings in more detail citing the scientific references.
Depending on whether the species is farmed or wild caught, you will be interested in different branches of the database.
Farm branch
Founded in 2013, the farm branch of the fair-fish database focuses on farmed aquatic species.
Catch branch
Founded in 2022, the catch branch of the fair-fish database focuses on wild-caught aquatic species.
The heart of the farm branch of the fair-fish database is the welfare assessment – or WelfareCheck | farm – resulting in the WelfareScore | farm for each species. The WelfareCheck | farm is a condensed assessment of the species' likelihood and potential for good welfare in aquaculture, based on welfare-related findings for 10 crucial criteria (home range, depth range, migration, reproduction, aggregation, aggression, substrate, stress, malformations, slaughter).
For those species with a Dossier, we conclude to-be-preferred farming conditions in the Advice | farm. They are not meant to be as detailed as a rearing manual but instead, challenge current farming standards and often take the form of what not to do.
In parallel to farm, the main element of the catch branch of the fair-fish database is the welfare assessment – or WelfareCheck | catch – with the WelfareScore | catch for each species caught with a specific catching method. The WelfareCheck | catch, too, is a condensed assessment of the species' likelihood and potential for good welfare – or better yet avoidance of decrease of good welfare – this time in fisheries. We base this on findings on welfare hazards in 10 steps along the catching process (prospection, setting, catching, emersion, release from gear, bycatch avoidance, sorting, discarding, storing, slaughter).
In contrast to the farm profiles, in the catch branch we assess the welfare separately for each method that the focus species is caught with. In the case of a species exclusively caught with one method, there will be one WelfareCheck, whereas in other species, there will be as many WelfareChecks as there are methods to catch the species with.
Summarising our findings of all WelfareChecks | catch for one species in Advice | catch, we conclude which catching method is the least welfare threatening for this species and which changes to the gear or the catching process will potentially result in improvements of welfare.
Try mousing over the element you are interested in - oftentimes you will find explanations this way. If not, there will be FAQ on many of the sub-pages with answers to questions that apply to the respective sub-page. If your question is not among those, contact us at ffdb@fair-fish.net.
It's right here! We decided to re-name it to fair-fish database for several reasons. The database has grown beyond dealing purely with ethology, more towards welfare in general – and so much more. Also, the partners fair-fish and FishEthoGroup decided to re-organise their partnership. While maintaining our friendship, we also desire for greater independence. So, the name "fair-fish database" establishes it as a fair-fish endeavour.