Information
Version: B | 1.1 (2022-07-20)
WelfareScore | farm
Condensed assessment of the species' likelihood and potential for good fish welfare in aquaculture, based on ethological findings for 10 crucial criteria.
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
- Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10)
General remarks
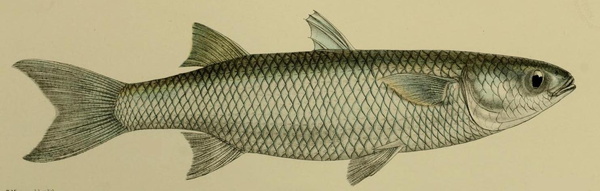
Mugil cephalus is an important food fish species which can be found in coastal tropical and subtropical waters worldwide. M. cephalus has been farmed for centuries in extensive and semi-intensive ponds in many countries and recently has been considered a promising species for aquaculture diversification. However, many behavioural aspects are yet to be fully understood, namely home range, migration, aggregation, aggression, malformations, and slaughter. Addressing these issues may improve the farming and welfare conditions.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
It is unclear for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a low amount of evidence.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of these circumstances?
It is low for minimal and high-standard farming conditions. Our conclusion is based on a high amount of evidence.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is unclear for minimal and high-standard farming conditions. Our conclusion is based on a low amount of evidence.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is unclear for minimal and high-standard farming conditions. Our conclusion is based on a low amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is unclear for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions. It is medium for high-standard farming conditions. Our conclusion is based on a medium amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
It is unclear for minimal and high-standard farming conditions. Our conclusion is based on a low amount of evidence.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
There are no findings for minimal and high-standard farming conditions.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 4 37, level 5 being fully domesticated.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes: WILD: carnivorous 38 39 40 41. FARM: fish meal and fish oil may be partly* replaced by non-forage fishery components for LARVAE 41 and JUVENILES, ADULTS, and SPAWNERS 42 43.
*partly = <51% – mostly = 51-99% – completely = 100%
Glossary
CATADROMOUS = migrating from fresh water into the sea to spawn
DOMESTICATION LEVEL 4 = entire life cycle closed in captivity without wild inputs 37
EURYHALINE = tolerant of a wide range of salinities
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
FRY = larvae from external feeding on, for details ➝ Findings 10.1 Ontogenetic development
IND = individuals
JUVENILES = fully developed but immature individuals, for details ➝ Findings 10.1 Ontogenetic development
LARVAE = hatching to mouth opening, for details ➝ Findings 10.1 Ontogenetic development
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
WILD = setting in the wild
Bibliography
2 Gehrke, Peter Charles, D. M. Gilligan, M Barwick, N. S. W. Fisheries, Cooperative Research Centre for Freshwater Ecology (Australia), and Sydney Catchment Authority. 2001. Fish communities and migration in the Shoalhaven River - before construction of a fishway. Article; Article/Report. Taylors Beach, NSW : NSW Fisheries Office of Conservation, Port Stephens Fisheries Centre.
3 Bacheler, Nathan M., Richard A. Wong, and Jeffrey A. Buckel. 2005. Movements and Mortality Rates of Striped Mullet in North Carolina. North American Journal of Fisheries Management 25: 361–373. https://doi.org/10.1577/M04-033.1.
4 De Silva, S. S., and E. I. L. Silva. 1979. Biology of young grey mullet, Mugil cephalus L., populatioins in a coastal lagoon in Sri Lanka. Journal of Fish Biology 15: 9–20. https://doi.org/10.1111/j.1095-8649.1979.tb03568.x.
5 Whitfield, A. K., J. Panfili, and J.-D. Durand. 2012. A global review of the cosmopolitan flathead mullet Mugil cephalus Linnaeus 1758 (Teleostei: Mugilidae), with emphasis on the biology, genetics, ecology and fisheries aspects of this apparent species complex. Reviews in Fish Biology and Fisheries 22: 641–681. https://doi.org/10.1007/s11160-012-9263-9.
6 Linder, Donald Ray, Kirk Strawn, and Richard W. Luebke. 1975. The culture of striped mullet (Mugil cephalus Linnaeus) in ponds receiving heated effluent from a power plant. Aquaculture 5: 151–161. https://doi.org/10.1016/0044-8486(75)90095-2.
7 Baliao, D. D., E. M. Rodriguez, and D. D. Gerochi. 1981. Culture of grey mullet, Mugil cephalus, Linnaeus in brackishwater ponds at two stocking densities. SEAFDEC Aquaculture Department Quarterly Research Report 5: 12–17.
8 Collins, Mark R., and Bruce W. Stender. 1989. Larval Striped Mullet (Mugil Cephalus) and White Mullet (Mugil Curema) off the Southeastern United States. Bulletin of Marine Science 45: 580–589.
9 Ditty, James G., and Richard F. Shaw. 1996. Spatial and Temporal Distribution of Larval Striped Mullet (Mugil Cephalus) and White Mullet (M. Curema, Family: Mugilidae) in the Northern Gulf of Mexico, with Notes on Mountain Mullet, Agonostomus Monticola. Bulletin of Marine Science 59: 271–288.
10 Harrison, I.J. 1995. Guia FAO para Identification de Especies para lo Fines de la Pesca. Pacifico Centro-Oriental. FAO. Vol. 3. Mugilidae. Lisas. Rome.
11 Camara, K. D., K. E. Carpenter, R. Djiman, F. Nunoo, A. Sagna, A. Sidibé, M. Sylla, et al. 2019. Flathead Mullet. e. T135567A127923853. The IUCN Red List of Threatened Species. International Union for Conservation of Nature.
12 Meynecke, Jan-Olaf, Geoffrey C. Poole, Jonathan Werry, and Shing Yip Lee. 2008. Use of PIT tag and underwater video recording in assessing estuarine fish movement in a high intertidal mangrove and salt marsh creek. Estuarine, Coastal and Shelf Science 79: 168–178. https://doi.org/10.1016/j.ecss.2008.03.019.
13 Whitfield, AJ, and SJM Blaber. 1978. Distribution, movements and fecundity of Mugilidae at Lake St. Lucia. The Lammergeyer 26: 53–63.
14 Saleh, M.A. 2006. Cultured Aquatic Species Information Programme. Mugil cephalus. Rome: FAO Fisheries and Aquaculture Department.
15 Arnold, Edgar L., and John R. Thompson. 1958. Offshore Spawning of the Striped Mullet, Mugil cephalus, in the Gulf of Mexico. Copeia 1958: 130–132. https://doi.org/10.2307/1440554.
16 Chang, Chih-Wei, Yoshiyuki Iizuka, and Wann-Nian Tzeng. 2004. Migratory environmental history of the grey mullet Mugil cephalus as revealed by otolith Sr:Ca ratios. Vol. 269. https://doi.org/10.3354/meps269277.
17 McDonough, Christopher J., and Charles A. Wenner. 2003. Growth, recruitment, and abundance of juvenile striped mullet (Mugil cephalus) in South Carolina estuaries. Fishery Bulletin 101: 343–357.
18 Thomson, JM. 1955. The Movements and Migrations of Mullet ( Mugil cephalus L.). Marine and Freshwater Research - MAR FRESHWATER RES 6. https://doi.org/10.1071/MF9550328.
19 Strydom, Nadine A., and Bruce D. d’Hotman. 2005. Estuary-dependence of larval fishes in a non-estuary associated South African surf zone: evidence for continuity of surf assemblages. Estuarine, Coastal and Shelf Science 63: 101–108. https://doi.org/10.1016/j.ecss.2004.10.013.
20 Hsu, Chih-Chieh, Chih-Wei Chang, Yoshiyuki Iizuka, and Wann-Nian Tzeng. 2009. A Growth Check Deposited at Estuarine Arrival in Otoliths of Juvenile Flathead Mullet (Mugil cephalus L.). Zoological Studies 48.
21 Nash, C. E., and Z.H. Shehadeh. 1980. Review of breeding and propagation techniques for grey mullet, Mugil cephalus L. 3. ICLARM Stud. Rev.
22 Lawson, Emmanuel, and Abayomi A.-A. Jimoh. 2010. Aspects of the biology of grey mullet, Mugil cephalus , in Lagos lagoon, Nigeria. Aquaculture, Aquarium, Conservation & Legislation 3.
23 Mohamadi, Mehrak, Gholam Reza Bishkoul, Abulhasan Rastiannasab, Hossein Khara, and Nasir Hut. 2014. Physiological indicators of salinity stress in the grey mullet, Mugil cephalus, Linnaeus, 1758 juveniles. Comparative Clinical Pathology 23: 1453–1456. https://doi.org/10.1007/s00580-013-1804-7.
24 Wallace, J. H., and R. P. van der Elst. 1975. The estuarine fishes of the East Coast of South Africa - (v. 1): Species composition and length distribution in the estuarine and marine environments.- Seasonal abundance and migrations.- (v. 2): Reproduction.- (v. 3): Occurrence of juveniles in estuaries.- Ecology, estuarine dependence and status.
25 Ochiai, Akira, and Susumu Umeda. 1969. Spawning Aspects of the Grey Mullet, Mugil cephalus L. Living on the Coastal Region of Kochi Prefecture. Japanese Journal of Ichthyology 16: 50–54. https://doi.org/10.11369/jji1950.16.50.
26 Ibáñez, A. L., and O. Gutiérrez Benítez. 2004. Climate variables and spawning migrations of the striped mullet and white mullet in the north-western area of the Gulf of Mexico. Journal of Fish Biology 65: 822–831. https://doi.org/10.1111/j.0022-1112.2004.00488.x.
27 R, Kurma Rao, and K. Ramesh Babu. 2016. Reproductive biology of the flathead grey mullet, Mugil cephalus (Linnaeus, 1758) from Krishna Estuarine Region, East Coast of Andhra Pradesh, India. International Journal of Fisheries and Aquatic Studies 4: 483–488.
28 Aizen, Joseph, Iris Meiri, Itai Tzchori, Berta Levavi-Sivan, and Hanna Rosenfeld. 2005. Enhancing spawning in the grey mullet (Mugil cephalus) by removal of dopaminergic inhibition. General and Comparative Endocrinology 142. 5th International Symposium on Fish Endocrynology: 212–221. https://doi.org/10.1016/j.ygcen.2005.01.002.
29 Meiri-Ashkenazi, I, V Zlatnikov, and H Rosenfeld. 2015. FSH agonist: The missing theraputic agent facilitating breeding for captive grey mullet (Mugil cephalus) broodstock. In . Rotterdam, Netherlands: European Aquaculture Society.
30 Kuo, Ching-Ming, Colin E. Nash, and Ziad H. Shehadeh. 1974. A procedural guide to induce spawning in grey mullet (Mugil cephalus L.). Aquaculture 3: 1–14. https://doi.org/10.1016/0044-8486(74)90094-5.
31 Gibbard, Gail L., Kirk Strawn, and David V. Aldrich. 1979. Feeding and Aggressive Behavior of Atlantic Croaker, Black Drum, and Striped Mullet in Monoculture and Polyculture. Proceedings of the World Mariculture Society 10: 241–248. https://doi.org/10.1111/j.1749-7345.1979.tb00023.x.
32 Eschmeyer, William N., and Earl S. Herald. 1983. Field Guide to Pacific Coast Fishes of North America: From the Gulf of Alaska to Baja California. Boston: Houghton Mifflin.
33 Mwandya, A. W., Y. D. Mgaya, M. C. Öhman, I. Bryceson, and M. Gullström. 2010. Distribution patterns of striped mullet Mugil cephalus in mangrove creeks, Zanzibar, Tanzania. African Journal of Marine Science 32: 85–93. https://doi.org/10.2989/18142321003714575.
34 Ako, Harry, Clyde S. Tamaru, Paul Bass, and Cheng-Sheng Lee. 1994. Enhancing the resistance to physical stress in larvae of Mugil cephalus by the feeding of enriched Artemia nauplii. Aquaculture 122: 81–90. https://doi.org/10.1016/0044-8486(94)90336-0.
35 Wanshu, Hong. 1992. Plasma cortisol and glucose concentrations in the striped mullet (Mugil cephalus L.) subjected to intense handling stress. Chinese Journal of Oceanology and Limnology 10: 40–43. https://doi.org/10.1007/BF02844298.
36 Nash, C. E., C-M. Kuo, W. D. Madden, and C. L. Paulsen. 1977. Swim bladder inflation and survival of Mugil cephalus to 50 days. Aquaculture 12: 89–94. https://doi.org/10.1016/0044-8486(77)90049-7.
37 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
38 De Silva, S. S., and M. J. S. Wijeyaratne. 1977. Studies on the biology of young grey mullet, Mugil cephalus L. II. Food and Feeding. Aquaculture 12: 157–167. https://doi.org/10.1016/0044-8486(77)90183-1.
39 FAO. 2018. Flathead grey mullet - Natural food and feeding habits.
40 Islam, Rafiqul, M. Belal Hossain, Ng Das, and Rashed-Un-Nabi Rafi. 2009. Food and feeding behaviour of grey mullet, Mugil cephalus (L.), of Bangladesh coastal water. Vol. 7.
41 Jamabo, NA, and NC Maduako. 2015. Food and Feeding Habits of grey mullet, Mugil Cephalus (Linnaeus, 1758) in Elichi Creek, Niger Delta, Nigeria. International Journal of Fisheries and Aquaculture 7: 25–29.
42 Wassef, E A, M H El Masry, and F R Mikhail. 2001. Growth enhancement and muscle structure of striped mullet, Mugil cephalus L., fingerlings by feeding algal meal-based diets. Aquaculture Research 32: 315–322. https://doi.org/10.1046/j.1355-557x.2001.00043.x.
43 Kalla, Alok, S K Garg, C P Kaushik, A R T Arasu, and G S Dinodia. 2018. Effect of replacement of fish meal with processed soybean on growth, digestibility and nutrient retention in Mugil cephalus (Linn.) fry.