
Information
Version: C | 2.0 (2023-09-08)
- profile update resulting in major editorial and content changes (changing the scoring in criteria 1-3, 6, 10)
- transfer to consistent age class and label structure resulting in changed appearance
WelfareScore | farm
Condensed assessment of the species' likelihood and potential for good fish welfare in aquaculture, based on ethological findings for 10 crucial criteria.
- Li = Likelihood that the individuals of the species experience good welfare under minimal farming conditions
- Po = Potential of the individuals of the species to experience good welfare under high-standard farming conditions
- Ce = Certainty of our findings in Likelihood and Potential
WelfareScore = Sum of criteria scoring "High" (max. 10)
General remarks
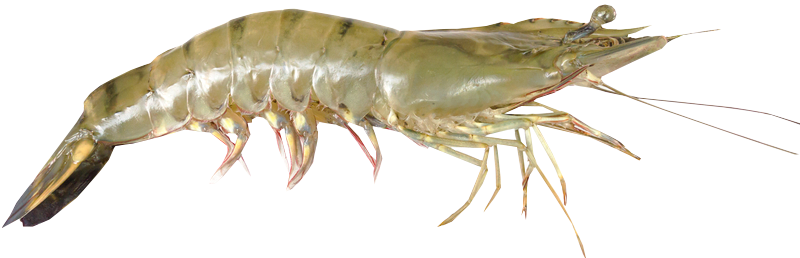
Penaeus vannamei is a shrimp species that naturally inhabits the eastern Pacific coast, including Mexico, Parita Gulf, Panama, and Peru. It is commonly cultured in brackish water or under low salinity conditions, being considered a promising species that has been introduced to many countries outside its native range, to western Atlantic coast and Asia, including China and Thailand. Aquaculture of this species has rapidly expanded worldwide since the early 2000s, with Southeast Asia as an important producer region. Penaeus vannamei has some advantages for aquaculture, like its fast and good growth, a great tolerance to a wide range of water parameters and high stocking densities, high disease resistance, low protein requirements, and high survival rates. As a consequence, many countries are moving from Penaeus monodon to Penaeus vannamei as the main species in shrimp farming. Despite that, unnatural stocking densities, shallow tanks, absence of substrate in culture tanks, and the highly invasive practice of eyestalk ablation are major problems that hinder this species’ welfare in aquaculture. Additionally, some important aspects of its natural behaviours and needs are still missing. Providing soft substrate that allows the expression of natural behaviours such as burrowing and grazing, as well as reducing stocking densities are simple measures that should help improve both performance and welfare. Eyestalk ablation has been shown to be unnecessary to induce spawning and therefore should not be implemented.
Note: due to reaching maturity after the typical age and weight at slaughter, there is no age class "ADULTS" in the profile.
1 Home range
Many species traverse in a limited horizontal space (even if just for a certain period of time per year); the home range may be described as a species' understanding of its environment (i.e., its cognitive map) for the most important resources it needs access to.
What is the probability of providing the species' whole home range in captivity?
There are unclear findings for minimal and high-standard farming conditions, as the missing wild information does not allow a comparison with farming conditions. Our conclusion is based on a medium amount of evidence.


2 Depth range
Given the availability of resources (food, shelter) or the need to avoid predators, species spend their time within a certain depth range.
What is the probability of providing the species' whole depth range in captivity?
It is low for minimal farming conditions, as some ponds and tanks do not cover the whole range in the wild (even if unclear for LARVAE to JUVENILES). It is medium for high-standard farming conditions, as other ponds overlap with the (estimated) range in the wild, but ponds deep enough for SPAWNERS are unlikely. Our conclusion is based on a medium amount of evidence, as (more detailed) wild information is missing for LARVAE to JUVENILES.


3 Migration
Some species undergo seasonal changes of environments for different purposes (feeding, spawning, etc.), and to move there, they migrate for more or less extensive distances.
What is the probability of providing farming conditions that are compatible with the migrating or habitat-changing behaviour of the species?
It is low for minimal and high-standard farming conditions, as the species undertakes extensive migrations, and we cannot be sure that providing each age class with their respective environmental conditions will satisfy their urge to migrate or whether they need to experience the transition. Our conclusion is based on a high amount of evidence.


4 Reproduction
A species reproduces at a certain age, season, and sex ratio and possibly involving courtship rituals.
What is the probability of the species reproducing naturally in captivity without manipulation of theses circumstances?
It is low for minimal farming conditions, as the species is manipulated (eyestalk ablation) and may be taken from the wild. It is high for high-standard farming conditions, as natural breeding with farm-reared individuals is possible and verified for the farming context. Our conclusion is based on a high amount of evidence.


5 Aggregation
Species differ in the way they co-exist with conspecifics or other species from being solitary to aggregating unstructured, casually roaming in shoals or closely coordinating in schools of varying densities.
What is the probability of providing farming conditions that are compatible with the aggregation behaviour of the species?
It is low for minimal farming conditions, as densities in some ponds and raceways are potentially stress inducing (even if unclear for eggs, LARVAE, and SPAWNERS). It is medium for high-standard farming conditions, as densities in other ponds and raceways overlap with the range in the wild. Our conclusion is based on a medium amount of evidence, as wild information is missing in eggs, LARVAE, and SPAWNERS, and more farm information is missing on density-related stress.


6 Aggression
There is a range of adverse reactions in species, spanning from being relatively indifferent towards others to defending valuable resources (e.g., food, territory, mates) to actively attacking opponents.
What is the probability of the species being non-aggressive and non-territorial in captivity?
It is low for minimal farming conditions, as aggression is present in almost all age classes. It is medium for high-standard farming conditions, as providing enough food and innovations to reduce aggression (directing with light, adjusted particle size) potentially work, but need to be verified for the farming context. Our conclusion is based on a low amount of evidence.


7 Substrate
Depending on where in the water column the species lives, it differs in interacting with or relying on various substrates for feeding or covering purposes (e.g., plants, rocks and stones, sand and mud, turbidity).
What is the probability of providing the species' substrate and shelter needs in captivity?
It is low for minimal farming conditions, as almost all age classes of the species use substrate, but cement tanks, raceways or cages are devoid of it. It is high for high-standard farming conditions given earthen ponds or polyethylene tanks with enrichment for POST-LARVAE, JUVENILES, and SPAWNERS (at least between spawning events). Our conclusion is based on a high amount of evidence.


8 Stress
Farming involves subjecting the species to diverse procedures (e.g., handling, air exposure, short-term confinement, short-term crowding, transport), sudden parameter changes or repeated disturbances (e.g., husbandry, size-grading).
What is the probability of the species not being stressed?
It is low for minimal farming conditions, as the species is stressed (handling, abrupt changes in temperature, salinity, and light, etc.). It is medium for high-standard farming conditions, as improvements are easily imaginable, but need to be verified for the farming context. Our conclusion is based on a medium amount of evidence.


9 Malformations
Deformities that – in contrast to diseases – are commonly irreversible may indicate sub-optimal rearing conditions (e.g., mechanical stress during hatching and rearing, environmental factors unless mentioned in crit. 3, aquatic pollutants, nutritional deficiencies) or a general incompatibility of the species with being farmed.
What is the probability of the species being malformed rarely?
There are unclear findings for minimal and high-standard farming conditions, as some papers report malformations and others not. Our conclusion is based on a low amount of evidence, as more papers are needed to be able to score.


10 Slaughter
The cornerstone for a humane treatment is that slaughter a) immediately follows stunning (i.e., while the individual is unconscious), b) happens according to a clear and reproducible set of instructions verified under farming conditions, and c) avoids pain, suffering, and distress.
What is the probability of the species being slaughtered according to a humane slaughter protocol?
It is low for minimal farming conditions, as not finding anything about stunning/slaughter indicates that they probably die of asphyxia. It is medium for high-standard farming conditions, as electrical stunning potentially induces unconsciousness fast and kills while still unconscious, but needs to be verified for P. vannamei and the farming context. Our conclusion is based on a low amount of evidence exclusively from related species.


Side note: Domestication
Teletchea and Fontaine introduced 5 domestication levels illustrating how far species are from having their life cycle closed in captivity without wild input, how long they have been reared in captivity, and whether breeding programmes are in place.
What is the species’ domestication level?
DOMESTICATION LEVEL 4 80, level 5 being fully domesticated.
Side note: Forage fish in the feed
450-1,000 milliard wild-caught fishes end up being processed into fish meal and fish oil each year which contributes to overfishing and represents enormous suffering. There is a broad range of feeding types within species reared in captivity.
To what degree may fish meal and fish oil based on forage fish be replaced by non-forage fishery components (e.g., poultry blood meal) or sustainable sources (e.g., soybean cake)?
All age classes:
- WILD: omnivorous 57.
- FARM: fish meal and fish oil may be mostly* replaced by sustainable sources 82 83 67 84. POST-LARVAE: pond fertilisation will promote phytoplankton that serves as feed 13; split bamboo poles used as periphyton substrate reduced 19% of feed usage 16. Feeding on supplementary feed increased from POST-LARVAE to ADULTS 33. Replacing fish meal and fish oil by discards and fish waste from processing plants, from bycatch fisheries and from aquaculture industry is possible 13, but no replacement by non-forage fishery components or sustainable sources reported [personal communication Maia 2023]. Replacing fish meal mostly* or completely* by animal byproduct or plant meals should be possible 10. Fish meal may be partly* replaced by sustainable sources (Plukenetia volubilis cake), with even a better growth 22.
- LAB: fish meal may be partly* replaced by sustainable sources 40.
* partly = <51% – mostly = 51-99% – completely = 100%
Glossary
AMPHIDROMOUS = migrating between fresh water and sea independent of spawning
BENTHIC = living at the bottom of a body of water, able to rest on the floor
BIOFLOC = dense microbial communities growing in flocs 25
DOMESTICATION LEVEL 4 = entire life cycle closed in captivity without wild inputs 81
EURYHALINE = tolerant of a wide range of salinities
FARM = setting in farming environment or under conditions simulating farming environment in terms of size of facility or number of individuals
IND = individuals
JUVENILES = fully developed but immature individuals, for details ➝ Findings 10.1 Ontogenetic development
LAB = setting in laboratory environment
LARVAE = hatching to mouth opening, for details ➝ Findings 10.1 Ontogenetic development
MYSIS = third larval stage, for details ➝ Findings 10.1 Ontogenetic development
NAUPLII = first larval stage after hatching, for details ➝ Findings 10.1 Ontogenetic development
PELAGIC = living independent of bottom and shore of a body of water
PHOTOPERIOD = duration of daylight
PLANKTONIC = horizontal movement limited to hydrodynamic displacement
POST-LARVAE = fully developed individuals, beginning of external sex differentiation; for details ➝ Findings 10.1 Ontogenetic development
PROTOZOEA = second larval stage, for details ➝ Findings 10.1 Ontogenetic development
SPAWNERS = adults during the spawning season; in farms: adults that are kept as broodstock
SUB-ADULTS = juveniles transforming to fully mature adults, for details ➝ Findings 10.1 Ontogenetic development
WILD = setting in the wild
Bibliography
2 Gunter, Gordon, J. Y. Christmas, and Rosamond Killebrew. 1964. Some Relations of Salinity to Population Distributions of Motile Estuarine Organisms, with Special Reference to Penaeid Shrimp. Ecology 45: 181–185. https://doi.org/10.2307/1937124.
3 Cook, Harry L., and M. Alice Murphy. 1969. The Culture of Larval Penaeid Shrimp. Transactions of the American Fisheries Society 98: 751–754. https://doi.org/10.1577/1548-8659(1969)98[751:TCOLPS]2.0.CO;2.
4 Bailey-Brock, J.H., and S. M. Moss. 1992. Penaeid taxonomy, biology and zoogeography. In Marine shrimp culture: Principles and practices, 9–27. Elsevier.
5 Briggs, M. 2006. Cultured Aquatic Species Information Programme. Penaeus vannamei. Rome: FAO Fisheries and Aquaculture Department.
6 Tan, J., S. Luan, B. Cao, K. Luo, X. Meng, and J. Kong. 2019. Comparison of growth and reproduction performance of broodstock Pacific white shrimp Litopenaeus vannamei reared in oceanic and brackish water. Aquaculture Research 50: 1893–1902. https://doi.org/10.1111/are.14075.
7 Galal, A. M., M. M. Said, and S. A. Sharaf. 2022. Reproductive Studies on Preparation of Whiteleg Shrimp Litopenaeus vannamei Broodstock in Commercial Hatcheries. Journal of Animal, Poultry & Fish Production 11: 35–42. https://doi.org/10.21608/japfp.2022.284091.
8 Fast, Arlo Wade, and L James Lester. 1992. Marine shrimp culture: principles and practices. Vol. 23. Elsevier.
9 Balakrishnan, Gunalan, Soundarapandian Peyail, Kumaran Ramachandran, Anand Theivasigamani, Maheswaran Chokkaiah, and Pushparaj Nataraj. 2011. Growth of Cultured White Leg Shrimp Litopenaeus Vannamei (Boone 1931) In Different Stocking Density. Advances in Applied Science Research 2: 107–113.
10 Boyd, C. E., A. A. McNevin, P. Racine, H. Q. Tinh, H. N. Minh, R. Viriyatum, D. Paungkaew, and C. Engle. 2017. Resource Use Assessment of Shrimp, Litopenaeus vannamei and Penaeus monodon, Production in Thailand and Vietnam. Journal of the World Aquaculture Society 48: 201–226. https://doi.org/10.1111/jwas.12394.
11 Abdelrahman, H. A., A. Abebe, and C. E. Boyd. 2019. Influence of variation in water temperature on survival, growth and yield of Pacific white shrimp Litopenaeus vannamei in inland ponds for low-salinity culture. Aquaculture Research 50: 658–672. https://doi.org/10.1111/are.13943.
12 Junda, M. 2018. Development of Intensive Shrimp Farming, Litopenaeus vannamei In Land-Based Ponds: Production and Management. Journal of Physics: Conference Series 1028: 012020. https://doi.org/10.1088/1742-6596/1028/1/012020.
13 Cuéllar-Anjel, Jorge, Cornelio Lara, Vielka Morales, Abelardo Gracia, and Óscar García Suárez. 2010. Manual of Best Management Practices for White Shrimp Penaeus vannamei Farming. Panama: OIRSA-OSPESCA.
14 Kasan, N. A., A. N. Dagang, and M. I. Abdullah. 2018. Application of biofloc technology (BFT) in shrimp aquaculture industry. IOP Conference Series: Earth and Environmental Science 196: 012043. https://doi.org/10.1088/1755-1315/196/1/012043.
15 Quyen, N. T. K., H. V. Hien, Le N. D. Khoi, N. Yagi, and A. K. L. Riple. 2020. Quality Management Practices of Intensive Whiteleg Shrimp (Litopenaeus vannamei) Farming: A Study of the Mekong Delta, Vietnam. Sustainability 12: 4520. https://doi.org/10.3390/su12114520.
16 Amirtharaj, V.K.S., B. Ahilan, C.B.T. Rajagopalsamy, M. Rosalind George, and P. Jawahar. 2022. Effect of split bamboo substrate on periphyton and growth performances of Pacific white shrimp, Penaeus vannamei (Boone, 1931), in low saline groundwater culture. Applied Ecology and Environmental Research 20: 2471–2484. https://doi.org/10.15666/aeer/2003_24712484.
17 Sookying, Daranee, Fabio Soller D. Silva, D. Allen Davis, and Terrill R. Hanson. 2011. Effects of stocking density on the performance of Pacific white shrimp Litopenaeus vannamei cultured under pond and outdoor tank conditions using a high soybean meal diet. Aquaculture 319: 232–239. https://doi.org/10.1016/j.aquaculture.2011.06.014.
18 Parnes, S, E Mills, C Segall, S Raviv, C Davis, and A Sagi. 2004. Reproductive readiness of the shrimp Litopenaeus vannamei grown in a brackish water system. Aquaculture 236: 593–606. https://doi.org/10.1016/j.aquaculture.2004.01.040.
19 Fast, A. W. 1992. Penaeid growout systems: an overview. Developments in aquaculture and fisheries science 23: 345–353.
20 Voltolina, Domenico, Jorge E. Watson-Toscano, Emilio Romero-Beltrán, and Juan Manuel Audelo-Naranjo. 2013. Nitrogen Recycling in Closed Cultures of Litopenaeus vannamei (Boone 1931) with Different Artificial Substrates. The Israeli Journal of Aquaculture - Bamidgeh 65.2013.907.
21 Otoshi, Clete A., Scott S. Naguwa, Frank C. Falesch, and Shaun M. Moss. 2007. Shrimp behavior may affect culture performance at super-intensive stocking densities. Global Aquaculture Advocate March/April: 67–69.
22 Renteria, P., A. J. Vizcaíno, M. J. Sánchez-Muros, R. A. Santacruz-Reyes, M. I. Saez, D. Fabrikov, F. G. Barroso, and M. C. Vargas-García. 2022. Effect of Replacing Fishmeal with Plukenetia volubilis Cake on Growth, Digestive Enzymes, and Body Composition in Whiteleg Shrimp (Litopenaeus vannamei). Fishes 7: 244. https://doi.org/10.3390/fishes7050244.
23 Bray, W. A., and A. L. Lawrence. 1992. Reproduction of Penaeus species in captivity. Developments in aquaculture and fisheries science 23: 93–170.
24 Palacios, Elena, Ilie S. Racolta, and Acuacultores de la Paz. 1999. Spawning Frequency Analysis of Wild and Pond-Reared Pacific White Shrimp Penaeus vannamei Broodstock under Large-Scale Hatchery Conditions. Journal of the World Aquaculture Society 30: 180–191. https://doi.org/10.1111/j.1749-7345.1999.tb00865.x.
25 Schveitzer, Rodrigo, Rafael Arantes, Manecas Francisco Baloi, Patrícia Fóes S. Costódio, Luis Vinatea Arana, Walter Quadros Seiffert, and Edemar Roberto Andreatta. 2013. Use of artificial substrates in the culture of Litopenaeus vannamei (Biofloc System) at different stocking densities: Effects on microbial activity, water quality and production rates. Aquacultural Engineering 54: 93–103. https://doi.org/10.1016/j.aquaeng.2012.12.003.
26 Moctezuma, M. A., and B. F. Blake. 1981. Burrowing Activity in Penaeus Vannamei Boone from the Caimanero-Huizache Lagoon System on the Pacific Coast of Mexico. Bulletin of Marine Science 31: 312–317.
27 Rothlisberg, Peter C., John A. Church, and Andrew M. G. Forbes. 1983. Modelling the advection of vertically migrating shrimp larvae. Journal of Marine Research 41: 511–538. https://doi.org/10.1357/002224083788519759.
28 Dall, WHBJ, BJ Hill, PC Rothlisberg, DJ Sharples, and others. 1990. The biology of the Penaeidae. Advances in marine biology 27.
29 Medina-Reyna, C. E. 2001. Growth and emigration of white shimp, Litopenaeus vannamei, in the Mar Muerto Lagoon, Southern Mexico. Naga, the ICLARM Quarterly 24: 30–34.
30 Rivera-Velázquez, G., L. A. Soto, I. H. Salgado-Ugarte, and E. J. Naranjo. 2008. Growth, mortality and migratory pattern of white shrimp (Litopenaeus vannamei, Crustacea, Penaeidae) in the Carretas-Pereyra coastal lagoon system, Mexico. Revista de Biología Tropical 56: 523–533.
31 Wakida-Kusunoki, Armando T., Luis Enrique Amador-del Angel, Patricia Carrillo Alejandro, and Cecilia Quiroga Brahms. 2011. Presence of Pacific white shrimp Litopenaeus vannamei (Boone, 1931) in the Southern Gulf of Mexico. Aquatic Invasions 6: S139–S142. https://doi.org/10.3391/ai.2011.6.S1.031.
32 Perez-Velazquez, Martin, Mayra L. González-Félix, D. A. Davis, Luke A. Roy, and Xuezhi Zhu. 2013. Studies of the Thermal and Haline Influences on Growth and Survival of Litopenaeus vannamei and Litopenaeus setiferus. Journal of the World Aquaculture Society 44: 229–238. https://doi.org/10.1111/jwas.12028.
33 Varadharajan, D., and N. Pushparajan. 2013. Food and Feeding Habits of Aquaculture Candidate a Potential Crustacean of Pacific White Shrimp Litopenaeus Vannamei, South East Coast of India. J Aquac Res Development 4: 5. https://doi.org/10.4172/2155-9546.1000161.
34 Pontes, Cibele Soares, Maria de Fatima Arruda, Alexandre Augusto de Lara Menezes, and Patrícia Pereira de Lima. 2006. Daily activity pattern of the marine shrimp Litopenaeus vannamei (Boone 1931) juveniles under laboratory conditions. Aquaculture Research 37: 1001–1006. https://doi.org/10.1111/j.1365-2109.2006.01519.x.
35 Panutrakul, S., and W. Senanan. 2021. Abundance of introduced Pacific whiteleg shrimp Penaeus vannamei (Boone, 1931) along the east coast of Thailand. Aquatic Invasions 16: 750–770.
36 Chong-Robles, Jennyfers, Guy Charmantier, Viviane Boulo, Joel Lizárraga-Valdéz, Luis M. Enríquez-Paredes, and Ivone Giffard-Mena. 2014. Osmoregulation pattern and salinity tolerance of the white shrimp Litopenaeus vannamei (Boone, 1931) during post-embryonic development. Aquaculture 422–423: 261–267. https://doi.org/10.1016/j.aquaculture.2013.11.034.
37 Senanan, W., S. Panutrakul, P. Barnette, V. Manthachitra, S. Chavanich, A. R. Kapuscinski, N. Tangkrock-Olan, et al. 2010. Ecological risk assessemnt of an alien aquatic species: a case study of Litopenaeus vannamei (Pacific whiteleg shrimp) aquaculture in the Bangpakong river, Thailand. In Tropical Deltas and Coastal Zones: Food Production, Communities and Environment at the Land-Water Interface, ed. Chu T. Hoanh, Brian W. Szuster, Kam Suan-Pheng, Abdelbagi M. Ismail, and Andrew D. Noble, 9:64–79. Comprehensive Assessment of Water Management in Agriculture. UK: CAB International.
38 Kumlu, Metin, Serhat Türkmen, and Mehmet Kumlu. 2010. Thermal tolerance of Litopenaeus vannamei (Crustacea: Penaeidae) acclimated to four temperatures. Journal of Thermal Biology 35: 305–308. https://doi.org/10.1016/j.jtherbio.2010.06.009.
39 Ogle, John T., Kathy Beaugez, and Jeffrey M. Lotz. 1992. Effects of Salinity on Survival and Growth of Postlarval Penaeus vannamei. Gulf and Caribbean Research 8: 415–421. https://doi.org/10.18785/grr.0804.07.
40 Martínez-Antonio, E. M., I. S. Racotta, J. C. Ruvalcaba-Márquez, and F. Magallón-Barajas. 2019. Modulation of stress response and productive performance of Litopenaeus vannamei through diet. PeerJ 7: e6850. https://doi.org/10.7717/peerj.6850.
41 Araneda, Marcelo, Eduardo P. Pérez, and Eucario Gasca-Leyva. 2008. White shrimp Penaeus vannamei culture in freshwater at three densities: Condition state based on length and weight. Aquaculture 283: 13–18. https://doi.org/10.1016/j.aquaculture.2008.06.030.
42 Su, Yuepeng, Shen Ma, and Cuimei Feng. 2010. Effects of Salinity Fluctuation on the Growth and Energy Budget of Juvenile Litopenaeus vannamei at Different Temperatures. Journal of Crustacean Biology 30: 430–434. https://doi.org/10.1651/09-3269.1.
43 Yano, I., R. A. Kanna, R. N. Oyama, and J. A. Wyban. 1988. Mating behaviour in the penaeid shrimp Penaeus vannamei. Marine Biology 97: 171–175. https://doi.org/10.1007/BF00391299.
44 Misamore, Michael J., and Craig L. Browdy. 1996. Mating Behavior in the White Shrimps Penaeus setiferus and P. vannamei: A Generalized Model for Mating in Penaeus. Journal of Crustacean Biology 16: 61–70. https://doi.org/10.2307/1548931.
45 Kumlu, Metin, Serhat Türkmen, Mehmet Kumlu, and O. Tufan Eroldoğan. 2011. Off-season Maturation and Spawning of the Pacific White Shrimp Litopenaeus vannamei in Sub-tropical Conditions. Turkish Journal of Fisheries and Aquatic Sciences 11.
46 Wright, James. 2016. Seajoy’s ablation-free shrimp answers emerging welfare concern | The Advocate.
47 Seajoy. 2018. Seajoy! - Non Ablation. http://www.seajoy.com/index.php/sustainable/non-ablation. Accessed May 22.
48 Undercurrent News. 2016. Seajoy stops shrimp eyestalk ablation. Undercurrent News.
49 Leung-Trujillo, Joanna K., and A. L. Lawrence. 1985. The effect of eyestalk ablation on spermatophore and sperm quality in Penaeus vannamei. Journal of the World Mariculture Society 16: 258–266. https://doi.org/10.1111/j.1749-7345.1985.tb00208.x.
50 Taylor, J., L. Vinatea, R. Ozorio, R. Schuweitzer, and E. R. Andreatta. 2004. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture 233: 173–179. https://doi.org/10.1016/j.aquaculture.2003.09.034.
51 Colt, J., and J. JUGUENIN. 1992. Shrimp hatchery design: engineering considerations. Developments in aquaculture and fisheries science 23: 245–285.
52 Chamberlain, George. 2017. Personal communication.
53 Sturmer, Leslie, Samocha, T, and A. L. Lawrence. 1992. Intensification of penaeid nursery systems. In Marine Shrimp Culture: Principles and Practices. Elsevier.
54 Edwards, R.R.C. 1977. Field experiments on growth and mortality of Penaeus vannamei in a Mexican coastal lagoon complex. Estuarine and Coastal Marine Science 5: 107–121. https://doi.org/10.1016/0302-3524(77)90076-7.
55 Hsiao, Shyh-Min Tom. 2015. Method for guiding aquatic crustaceans by utilizing their biological tendency responding to bright and dark contrast. http://www.google.com/patents/US7000567. Accessed November 12.
56 Balboa, William A., Timothy L. King, and Paul C. Hammerschmidt. 1991. Occurrence of Pacific White Shrimp in Lower Laguna Madre, Texas. Proc. Annu. Conf. Southeast. Assoc. Fish. and Wildl. Agencies 45: 288–292.
57 Panutrakul, S., W. Senanan, S. Chavanich, N. Tangkrock-Olan, and V. Viyakarn. 2010. Ability of Litopenaeus vannamei to survive and compete with local marine shrimp species in the Bangpakong river, Thailand. In Tropical Deltas and Coastal Zones: Food Production, Communities and Environment at the Land-Water Interface, ed. Chu T. Hoanh, Brian W. Szuster, Kam Suan-Pheng, Abdelbagi M. Ismail, and Andrew D. Noble, 9:80–92. Comprehensive Assessment of Water Management in Agriculture. UK: CAB International.
58 Roque, Ana. 2015. Personal communication.
59 Obaldo, Leonard G., and Reiji Masuda. 2006. Effect of Diet Size on Feeding Behavior and Growth of Pacific White Shrimp, Litopenaeus vannamei. Journal of Applied Aquaculture 18: 101–110. https://doi.org/10.1300/J028v18n01_07.
60 Gatune, Charles, Ann Vanreusel, Clio Cnudde, Renison Ruwa, Peter Bossier, and Marleen De Troch. 2012. Decomposing mangrove litter supports a microbial biofilm with potential nutritive value to penaeid shrimp post larvae. Journal of Experimental Marine Biology and Ecology 426–427: 28–38. https://doi.org/10.1016/j.jembe.2012.05.015.
61 Salim, G., A. Indarjo, M. Zein, L. Y. Prakoso, Suhirwan, GS A. Daengs, and Rukisah. 2020. The allometric growth and condition index comparison of white shrimp (Litopenaeus vannamei) from fishpond and juata laut waters, Tarakan (Indonesia). IOP Conference Series: Earth and Environmental Science 564: 012009. https://doi.org/10.1088/1755-1315/564/1/012009.
62 Luchiari, A. C., A. O. Marques, and F. A. M. Freire. 2012. Effects of substrate colour preference on growth of the shrimp Litopenaeus vannamei (Boone, 1931) (Decapoda, Penaeoidea). Crustaceana 85: 789–800. https://doi.org/10.1163/156854012X650232.
63 Santos, Daniele Bezerra dos, Cibele Soares Pontes, Fúlvio Aurélio Morais Freire, and Ambrósio Paula Bessa Júnior. 2011. Efeito do tipo de sedimento na eficiência alimentar, crescimento e sobrevivência de Litopenaeus vannamei (Boone, 1931). Acta Scientiarum. Biological Sciences 33: 369–375. https://doi.org/10.4025/actascibiolsci.v33i4.6134.
64 Ritvo, G, T.M Samocha, A.L Lawrence, and W.H Neill. 1998. Growth of Penaeus vannamei on soils from various Texas shrimp farms, under laboratory conditions. Aquaculture 163: 101–110. https://doi.org/10.1016/S0044-8486(98)00225-7.
65 Hirono, Yosuke, and Mark Leslie. 1992. Chapter 38 - Shrimp culture industry in Ecuador. In Marine Shrimp Culture, ed. Arlo W. Fast and L. James Lester, 783–815. Developments in Aquaculture and Fisheries Science. Amsterdam: Elsevier. https://doi.org/10.1016/B978-0-444-88606-4.50044-8.
66 McGraw, W. J., D. A. Davis, D. Teichert-Coddington, and D. B. Rouse. 2002. Acclimation of Litopenaeus vannamei Postlarvae to Low Salinity: Influence of Age, Salinity Endpoint, and Rate of Salinity Reduction. Journal of the World Aquaculture Society 33: 78–84.
67 Jayasankar, Vidya, Safiah Jasmani, Takeshi Nomura, Setsuo Nohara, Do Thi Thanh Huong, and Marcy N. Wilder. 2009. Low Salinity Rearing of the Pacific White Shrimp Litopenaeus vannamei: Acclimation, Survival and Growth of Postlarvae and Juveniles. Japan Agricultural Research Quarterly: JARQ 43: 345–350. https://doi.org/10.6090/jarq.43.345.
68 Mercier, Laurence, Ilie S. Racotta, Gloria Yepiz‐Plascencia, Adriana Muhlia‐Almazán, Roberto Civera, Marcos F. Quiñones‐Arreola, Mathieu Wille, Patrick Sorgeloos, and Elena Palacios. 2009. Effect of diets containing different levels of highly unsaturated fatty acids on physiological and immune responses in Pacific whiteleg shrimp Litopenaeus vannamei (Boone) exposed to handling stress. Aquaculture Research 40: 1849–1863. https://doi.org/10.1111/j.1365-2109.2009.02291.x.
69 Aparicio-Simón, Benjamin, Manuel Piñón, Radu Racotta, and Ilie S. Racotta. 2010. Neuroendocrine and metabolic responses of Pacific whiteleg shrimp Litopenaeus vannamei exposed to acute handling stress. Aquaculture 298: 308–314. https://doi.org/10.1016/j.aquaculture.2009.10.016.
70 Mercier, Laurence, Elena Palacios, Ángel I. Campa-Córdova, Dariel Tovar-Ramírez, Roberto Hernández-Herrera, and Ilie S. Racotta. 2006. Metabolic and immune responses in Pacific whiteleg shrimp Litopenaeus vannamei exposed to a repeated handling stress. Aquaculture 258: 633–640. https://doi.org/10.1016/j.aquaculture.2006.04.036.
71 Racotta, Ilie S., and Elena Palacios. 1998. Hemolymph Metabolic Variables in Response to Experimental Manipulation Stress and Serotonin Injection in Penaeus vannamei. Journal of the World Aquaculture Society 29: 351–356. https://doi.org/10.1111/j.1749-7345.1998.tb00658.x.
72 Guo, Biao, Fang Wang, Ying Li, and Shuanglin Dong. 2013. Effect of periodic light intensity change on the molting frequency and growth of Litopenaeus vannamei. Aquaculture 396–399: 66–70. https://doi.org/10.1016/j.aquaculture.2013.02.033.
73 Sainz-Hernández, Juan Carlos, Ilie S. Racotta, Silvie Dumas, and Jorge Hernández-López. 2008. Effect of unilateral and bilateral eyestalk ablation in Litopenaeus vannamei male and female on several metabolic and immunologic variables. Aquaculture 283: 188–193. https://doi.org/10.1016/j.aquaculture.2008.07.002.
74 Sánchez-Barajas, Maximiliano, Marco Agustín Liñán-Cabello, and Alfredo Mena-Herrera. 2009. Detection of yellow-head disease in intensive freshwater production systems of Litopenaeus vannamei. Aquaculture International 17: 101–112. https://doi.org/10.1007/s10499-008-9183-9.
75 Neil, Douglas. 2010. The effect of the CrustastunTM on nerve activity in crabs and lobsters. Scientific Report to Studham Technologies Ltd. UK: University of Glasgow.
76 Bickmeyer, Ulf, and Torsten Fregin. 2015. Vergleichende Untersuchungen zur tiergerechten Betäubung oder Tötung von Krustentieren. Bremerhaven: Alfred Wegener Institut, Helmholtz Zentrum für Polar- und Meeresforschung.
77 Neil, Douglas, and John Thompson. 2012. The Stress Induced by the CrustastunTM Process in Two Commercially Important Decapod Crustaceans: The Edible Brown Cancer Pagurus and the European Lobster Homarus Gammarus. Project report. UK: University of Glasgow.
78 Sparrey, Julian. 2005. Testing of Crustastun single crab and lobster stunner. Unpublished research report. UK.
79 Roth, B, and S Øines. 2010. Stunning and killing of edible crabs (Cancer pagurus). Animal Welfare 19: 287–294.
80 Teletchea, Fabrice. 2015. Domestication of Marine Fish Species: Update and Perspectives. Journal of Marine Science and Engineering 3: 1227–1243. https://doi.org/10.3390/jmse3041227.
81 Teletchea, Fabrice, and Pascal Fontaine. 2012. Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15: 181–195. https://doi.org/10.1111/faf.12006.
82 Browdy, Craig, Gloria Seaborn, Heidi Atwood, D. Allen Davis, Robert A. Bullis, Tzachi M. Samocha, Ed Wirth, and John W. Leffler. 2006. Comparison of Pond Production Efficiency, Fatty Acid Profiles, and Contaminants in Litopenaeus vannamei Fed Organic Plant-based and Fish-meal-based Diets. Journal of the World Aquaculture Society 37: 437–451. https://doi.org/10.1111/j.1749-7345.2006.00057.x.
83 Amaya, Elkin A., D. Allen Davis, and David B. Rouse. 2007. Replacement of fish meal in practical diets for the Pacific white shrimp (Litopenaeus vannamei) reared under pond conditions. Aquaculture 262: 393–401. https://doi.org/10.1016/j.aquaculture.2006.11.015.
84 Samocha, T. 2004. Substitution of fish meal by co-extruded soybean poultry by-product meal in practical diets for the Pacific white shrimp, Litopenaeus vannamei. Aquaculture 231: 197–203. https://doi.org/10.1016/j.aquaculture.2003.08.023.